| CPC C07D 239/47 (2013.01) [A61K 31/4545 (2013.01); A61K 31/513 (2013.01); A61K 31/5377 (2013.01); A61K 31/551 (2013.01); C07B 59/002 (2013.01); C07D 239/36 (2013.01); C07D 401/04 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 403/12 (2013.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 471/10 (2013.01); C07B 2200/05 (2013.01)] | 4 Claims |
|
1. A method for treating cancer selected from the group consisting of acute myeloid leukemia (AML), breast cancer, and prostate cancer in a subject in need thereof comprising administering to the subject a therapeutically effective dose of a compound of Formula (I), or a pharmaceutically acceptable salt thereof:
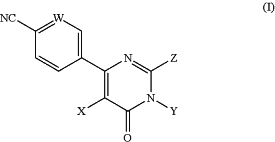 wherein:
W is N, C—H, or C—F;
Y is hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, or optionally substituted cycloalkylalkyl; and
(i) X is hydrogen, halogen, —CN, optionally substituted alkyl, optionally substituted alkynyl, optionally substituted carbocyclylalkynyl, or optionally substituted heteroaryl; and
Z is an optionally substituted group chosen from alkyl, carbocyclyl, C-attached heterocyclyl, N-attached heterocyclyl, heterocyclylalkyl, heterocyclylalkenyl, —O-heterocyclyl, —N(R)-heterocyclyl, —O-heterocyclylalkyl, —N(R)-heterocyclylalkyl, —N(R)(C1-C4alkylene)-NR2, or —O(C1-C4alkylene)-NR2; wherein R is hydrogen or C1-C4alkyl; or
(ii) X is optionally substituted aryl; and
Z is an optionally substituted group chosen from C-attached heterocyclyl, heterocyclylalkyl, heterocyclylalkenyl, —O-heterocyclyl, —N(R)-heterocyclyl, —O—heterocyclylalkyl, —N(R)-heterocyclylalkyl, —N(R)(C1-C4alkylene)-NR2, or —O(C1-C4alkylene)-NR2; wherein R is hydrogen or C1-C4alkyl.
|