| CPC B29C 64/379 (2017.08) [B29C 64/268 (2017.08); B29C 64/321 (2017.08); B29C 64/393 (2017.08); B33Y 40/00 (2014.12); B33Y 70/00 (2014.12)] | 12 Claims |
|
1. A bioprinting system for producing biological tissue, comprising:
a) a printing module containing at least one printhead for printing transferable objects of biological interest and at least one target in a controlled manner for producing a biological tissue;
b) a feed source for the printhead for printing objects of biological interest;
c) an activation device configured to transfer the objects of biological interest to the target;
d) a displacement device configured to cause relative displacement of the printhead with respect to the target; and
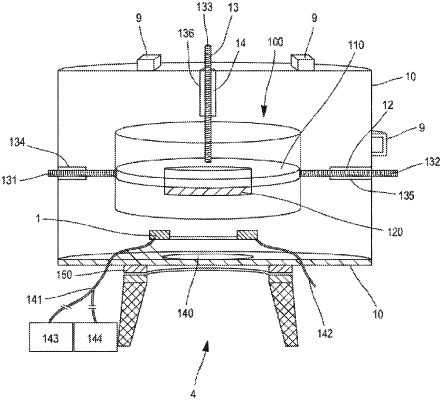
e) a sterilizable sealed enclosure defining a jacket of the printing module, the sterilizable sealed enclosure comprising a movement means, the movement means comprising a magnetic target support interacting with stator coils outside the sterilizable sealed enclosure;
wherein
the printing module is located within the sterilizable sealed enclosure;
the activation device is located outside the sterilizable sealed enclosure and physically separable from the printhead, and mechanically, electrically, acoustically or optically coupled with the printhead, the activation device configured to control the physical transfer of the object of biological interest present in the printhead to the target; and
the sterilizable sealed enclosure has a leaktight interaction zone with the activation device.
|