| CPC A61L 31/16 (2013.01) [A61L 31/10 (2013.01); A61L 31/148 (2013.01); A61L 2300/21 (2013.01); A61L 2300/216 (2013.01); A61L 2300/41 (2013.01); A61L 2300/416 (2013.01); A61L 2300/626 (2013.01); A61L 2300/802 (2013.01); A61L 2420/02 (2013.01); A61L 2420/06 (2013.01)] | 18 Claims |
|
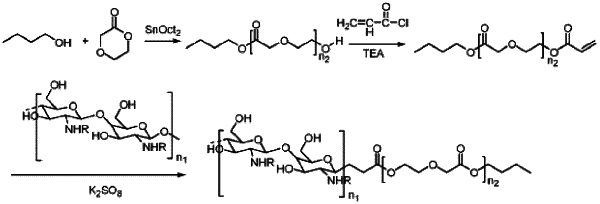
1. A preparation method of a polymer drug-loaded micellar solution of an absorbable vascular stent coating for angiostenosis in an infant, comprising the following steps:
S1: dissolving a drug to be encapsulated in an appropriate amount of an emulsifying agent, adding a chitosan-poly(p-dioxanone) amphiphilic block copolymer (chitosan-b-PPDO copolymer), and thoroughly mixing to obtain a drug-copolymer solution, wherein a mass ratio of the drug to be encapsulated to the chitosan-b-PPDO copolymer is 10:(20-60),
S2: adding the drug-copolymer solution obtained in S1 dropwise to an emulsifying agent aqueous solution prepared in advance, and continuously stirring for 12 h to 36 h to obtain a stable drug-loaded micellar solution, wherein a mass ratio of an emulsifying agent to water in the emulsifying agent aqueous solution is 1:(0.5-2); a mass of the drug-copolymer solution is 2% to 10% of a mass of the emulsifying agent aqueous solution; and
S3: removing the emulsifying agent from the stable drug-loaded micellar solution obtained in S2 through a vacuum evaporation, stirring a resulting concentrate for 2 h to 4 h, and centrifuging the resulting concentrate to obtain a supernatant; and filtering the supernatant to obtain a filtrate, and subjecting the filtrate to a dialysis to completely remove an unbound copolymer and a residual emulsifying agent to obtain the polymer drug-loaded micellar solution.
|