| CPC A61K 9/2077 (2013.01) [A61K 9/2027 (2013.01); A61K 9/2054 (2013.01); A61K 9/2059 (2013.01); A61K 31/422 (2013.01); A61K 31/44 (2013.01); A61K 31/4439 (2013.01); A61K 31/4468 (2013.01); A61K 31/496 (2013.01); A61K 31/4985 (2013.01); A61K 31/506 (2013.01); A61K 31/519 (2013.01); A61K 31/5377 (2013.01); A61K 31/7072 (2013.01)] | 18 Claims |
|
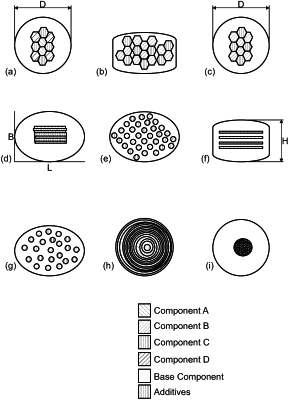
1. A drug delivery system for a controlled administration of an active pharmaceutical ingredient, API, to a body, the system comprising:
a base component soluble in body fluids,
a separate first component soluble in body fluids, and
a separate second component soluble in body fluids,
wherein the first component comprises a therapeutically effective amount of a first API,
wherein the second component comprises a therapeutically effective amount of a second API,
wherein the first component and the second component are inhomogeneously arranged in the base component,
wherein the first component or a separate marking component is optically different from the base component and arranged such that it forms a two-dimensional pattern on the surface of the system.
|