| CPC A61K 47/55 (2017.08) [C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 519/00 (2013.01)] | 19 Claims |
|
1. A bifunctional compound having the chemical structure:
ULM-L-PTM,
or a pharmaceutically acceptable salt, enantiomer, or stereoisomer thereof,
wherein:
(a) the ULM is a Von Hippel-Lindau (VHL) ligase-binding moiety (VLM) with a chemical structure represented by:
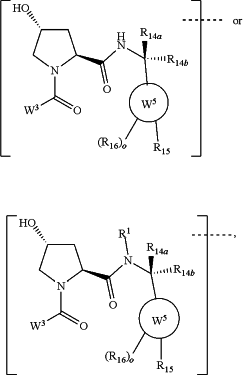 wherein:
W3 is selected from the group of an optionally substituted aryl, optionally substituted heteroaryl, or
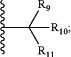 R9 and R10 are independently hydrogen, optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted hydroxyalkyl, optionally substituted heteroaryl, or haloalkyl, or R9, R10, and the carbon atom to which they are attached form an optionally substituted cycloalkyl;
R11 is selected from the group of an optionally substituted heterocyclic, optionally substituted alkoxy, optionally substituted heteroaryl, optionally substituted aryl,
 R12 is selected from the group of H or optionally substituted alkyl;
R13 is selected from the group of H, optionally substituted alkyl, optionally substituted alkylcarbonyl, optionally substituted (cycloalkyl)alkylcarbonyl, optionally substituted aralkylcarbonyl, optionally substituted arylcarbonyl, optionally substituted (heterocyclyl)carbonyl, or optionally substituted aralkyl;
R1 is H, linear or branched C1-C6 alkyl group optionally substituted by 1 or more halo or —OH groups;
R14a, R14b, are each independently selected from the group of H, haloalkyl, optionally substituted alkoxy, (CH2)m′OCOCH2(CH2)m′OCH2(CH2)m, CO(CH2)m, OH, (CH2)m′OCOCH2(CH2)m′CO(CH2)m′OH, optionally substituted hydroxyl alkyl, —(CH2)m′C(═O)(CH2)m′C(═O)(OH), or optionally substituted alkyl optionally with one or more carbons replaced with an oxygen;
each m′ is individually an integer from 1 to 4;
W5 is selected from the group of an optionally substituted phenyl or an optionally substituted 5-10 membered heteroaryl,
R15 is selected from the group of H, halogen, CN, OH, NO2, NR14aR14b, OR14a, CONR14aR14b, NR14aCOR14b, SO2NR14aR14b, NR14a SO2R14b, optionally substituted alkyl, optionally substituted haloalkyl, optionally substituted haloalkoxy, optionally substituted aryl, optionally substituted heteroaryl, optionally substituted cycloalkyl, or optionally substituted cycloheteroalkyl;
each R16 is independently selected from the group of H, CN, halo, optionally substituted alkyl optionally having one or more carbon atoms replaced with an oxygen atom, optionally substituted haloalkyl, hydroxy, or optionally substituted haloalkoxy;
o is 0, 1, 2, 3, or 4;
R18 is independently selected from the group of H, halo, optionally substituted alkoxy, cyano, optionally substituted alkyl, haloalkyl, haloalkoxy or a linker; and
p is 0, 1, 2, 3, or 4; and
the dashed line indicates the site of attachment the chemical linker group (L) coupling the ULM to the PTM;
(b) the PTM is a small molecule comprising a rapidly accelerated fibrosarcoma (RAF) protein targeting moiety wherein the PTM is represented by chemical structure PTM-IIa or PTM-IIb:
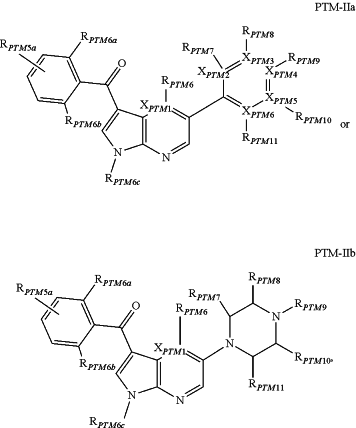 wherein:
XPTM1, XPTM2, XPTM3, XPTM4, XPTM5, and XPTM6 are independently selected from CH or N;
RPTM5a is selected from the group consisting of: H, optionally substituted —C(O)NH2, optionally substituted
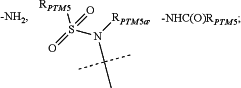 RPTM5 is selected from the group consisting of
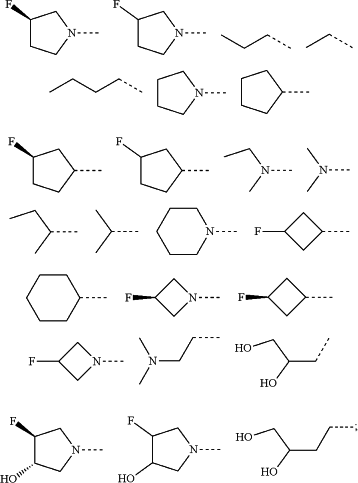  RPTM5b is hydrogen or a linear or branched C1-C4 alkyl;
RPTM6a and RPTM6b are each independently selected from hydrogen, halogen, or optionally substituted linear or branched C1-C6 alkyl;
RPTM6 is either of the following groups: absent, hydrogen, halogen, aryl, methyl, ethyl, OCH3, NHCH3 or M1-CH2—CH2-M2, wherein M1 is CH2, O and NH, and M2 is hydrogen, alkyl, cyclic alkyl, aryl or heterocycle;
RPTM6c is hydrogen or a linear or branched C1-C4 alkyl;
RPTM7 is absent, hydrogen, halogen, aryl, methyl, ethyl, OCH3, NHCH3 or M1-CH2—CH2-M2, wherein M1 is CH2, O and NH, and M2 is hydrogen, alkyl, cyclic alkyl, aryl or heterocycle;
RPTM8, RPTM9 or RPTM10 are independently selected from the group consisting of absent, hydrogen, halogen, aryl, heteroaryl, alkyl, cycloalkyl, heterocycle, methyl, ethyl, OCH3, NHCH3 or M1-CH2—CH2-M2, wherein M1 is CH2, O and NH, and M2 is hydrogen, alkyl, cyclic alkyl, aryl or heterocycle; and
RPTM11 is absent, hydrogen, halogen, methyl, ethyl, OCH3, NHCH3 or M1-CH2—CH2-M2 in which M1, wherein CH2, O and NH, and M2 is hydrogen, alkyl, cyclic alkyl, aryl or heterocycle;
at least one of RPTM8, RPTM9, or RPTM10 is modified to be covalently joined to a chemical linker group (L) coupling the PTM to the ULM, or two of RPTM8, RPTM9, and RPTM10 are modified to form a polycyclic (e.g., bicyclic) fused ring with a chemical linker group (L) coupling the PTM to the ULM; and
(c) the L is a chemical linking moiety connecting the ULM and the PTM, wherein the linker group comprises a chemical structural unit represented by the formula:
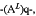 wherein:
(AL)q is a group which is connected to at least one of the ULM, the PTM, or a combination thereof;
q is an integer greater than or equal to 1;
each AL is independently selected from the group consisting of, a bond, CRL1RL2, O, S, SO, SO2, NRL3, SO2NRL3, SONRL3, CONRL3, NRL3CONRL4, NRL3SO2NRL4, CO, CRL1═CRL2, C≡C, C3-11cycloalkyl optionally substituted with 0-6 RL1 and/or RL2 groups, C3-11heterocyclyl optionally substituted with 0-6 RL1 and/or RL2 groups, aryl optionally substituted with 0-6 RL1 and/or RL2 groups, heteroaryl optionally substituted with 0-6 RL1 and/or RL2 groups, where RL1 or RL2, each independently are optionally linked to other groups to form cycloalkyl and/or heterocyclyl moiety, optionally substituted with 0-4 RL5 groups; and
RL1, RL2, RL3, RL4 and RL5 are, each independently, H, halo, C1-8alkyl, OC1-8alkyl, SC1-8alkyl, NHC1-8alkyl, N(C1-8alkyl)2, C3-11 cycloalkyl, aryl, heteroaryl, C3-11heterocyclyl, OC3-8cycloalkyl, SC3-8cycloalkyl, NHC3-8cycloalkyl, N(C3-8cycloalkyl)2, N(C3-8cycloalkyl)(C1-8alkyl), OH, NH2, SH, SO2C1-8alkyl, CCH, CH═CH(C1-8alkyl), C(C1-8alkyl)═CH(C1-8alkyl), C(C1-8alkyl)═C(C1-8alkyl)2, COC1-8alkyl, CO2H, halogen, CN, CF3, CHF2, CH2F, NO2, SF5, SO2NHC1-8alkyl, SO2N(C1-8alkyl)2, SONHC1-8alkyl, SON(C1-8alkyl)2, CONHC1-8alkyl, CON(C1-8alkyl)2, N(C1-8alkyl)CONH(C1-8alkyl).
|