| CPC A61K 38/4813 (2013.01) [A61K 47/10 (2013.01); A61K 47/12 (2013.01); A61K 47/26 (2013.01); A61K 47/38 (2013.01); A61P 31/14 (2018.01)] | 14 Claims |
|
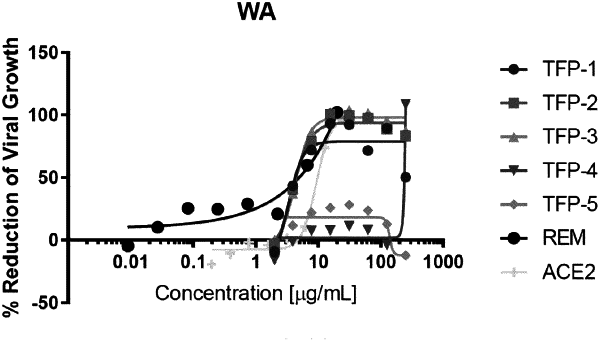
1. A method of treating a subject infected with or at risk of being infected with a coronavirus, the method comprising administering to the subject a therapeutically effective amount of a formulation comprising:
(a) a recombinant angiotensin converting enzyme 2 (rACE-2) protein that specifically binds to a coronavirus protein, wherein the rACE-2 protein comprises an amino acid sequence with at least 90% sequence identity to SEQ ID NO:2;
(b) a cellulose derivative selected from hydroxypropyl cellulose (HPC), hydroxypropyl methyl cellulose (HPMC), or a combination thereof; and
(c) an excipient selected from a glycol alcohol, a sugar alcohol, an acid, an ester, or any combination thereof, wherein the rACE-2 protein is at a concentration of about 2 percent weight/weight (w/w).
|