| CPC A61K 38/1793 (2013.01) [A61K 45/06 (2013.01); A61P 35/00 (2018.01); C07K 16/2803 (2013.01); C07K 16/2818 (2013.01); A61K 2039/505 (2013.01)] | 44 Claims |
|
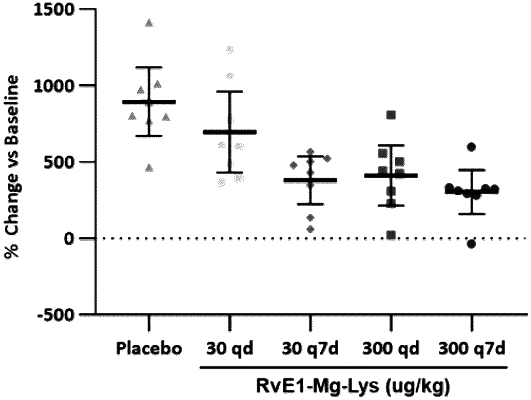
1. A method for treating cancer in a subject in need of such treatment, the method comprising administering to the subject a pharmaceutical composition comprising a mineral amino acid salt of resolvin E1 (“RvE1”) in a therapeutic regimen of every six days (Q6D) or every seven days (Q7D), either alone as monotherapy or as adjuvant or neo-adjuvant therapy, optionally in combination with one or more additional therapeutic agents or therapies, wherein the method does not comprise daily administration of RvE1 and wherein the mineral amino acid salt has a structure of Formula IV, or an enantiomer, polymorph, solvate, or hydrate thereof:
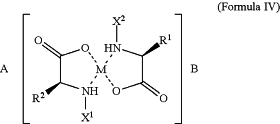 wherein
M is a divalent metal selected from magnesium (Mg2+), calcium (Ca2+), and zinc (Zn2+);
A and B are each RvE1;
R1 and R2 are each independently a C1-C10 alkyl comprising at least one basic function; and
X1 and X2 are each independently H or CO—Z and Z is a peptide comprising 1 to 5 amino acids.
|