| CPC A61K 38/07 (2013.01) [A61K 9/1617 (2013.01); A61K 9/1623 (2013.01); A61K 9/1694 (2013.01); A61K 9/4808 (2013.01); A61K 9/5089 (2013.01); A61K 31/40 (2013.01); A61K 31/485 (2013.01)] | 11 Claims |
|
1. A formulation for oral delivery of a kappa opioid receptor agonist, the formulation comprising,
particles including a kappa opioid receptor agonist and an oligosaccharide, wherein the kappa opioid receptor agonist is CR845 having the formula:
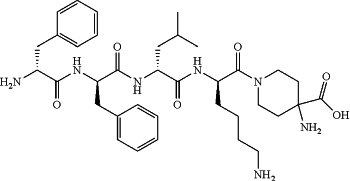 D-Phe-D-Phe-D-Leu-D-Lys-[ω(4-aminopiperidine-4-carboxylic acid)]-OH or a hydrate, salt, acid salt or acid salt hydrate thereof;
wherein the oligosaccharide is trehalose and wherein the CR845 is stable for at least a year at 25° C.
|