| CPC A61K 35/28 (2013.01) [A01N 1/0221 (2013.01); A61K 35/50 (2013.01); A61K 38/1825 (2013.01); A61K 38/1841 (2013.01); A61K 38/39 (2013.01); A61K 38/57 (2013.01); C12N 5/0605 (2013.01); C12N 2500/02 (2013.01); C12N 2501/115 (2013.01); C12N 2502/025 (2013.01)] | 4 Claims |
|
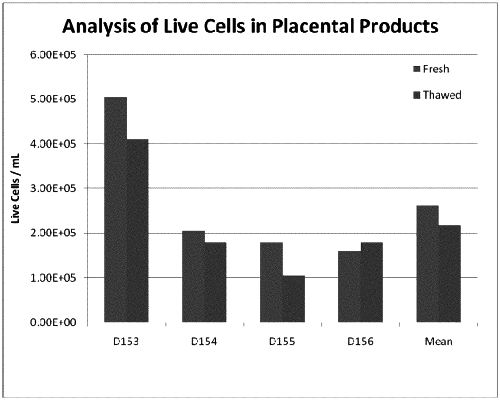
1. A previously refrigerated and cryopreserved placental product comprising:
a. a first fraction of placental cells isolated from a first portion of a chorionic membrane, the first fraction of placental cells comprising at least 70% viable cells of the cryopreserved placental product after thawing,
wherein the placental cells comprise at least two cell types comprising stromal cells and placental stem cells, and
wherein the first fraction of placental cells is not expanded ex vivo;
b. a disrupted chorionic membrane derived from a second portion of the chorionic membrane, the disrupted chorionic membrane comprising pieces of native chorionic membrane tissue that are greater than 100 μm in length, and a second fraction of placental cells,
wherein the disrupted chorionic membrane is depleted in functional CD14+ macrophages;
and
c. one or more placental factors comprised in or released by the first fraction of placental cells, the pieces of native chorionic membrane tissue, and the second fraction of placental cells,
wherein the placental product was refrigerated in a cryopreservation medium at a temperature of 2-8° C. for about 30 minutes to about 60 minutes prior to being cryopreserved.
|