| CPC A61K 31/785 (2013.01) [C08F 226/02 (2013.01)] | 21 Claims |
|
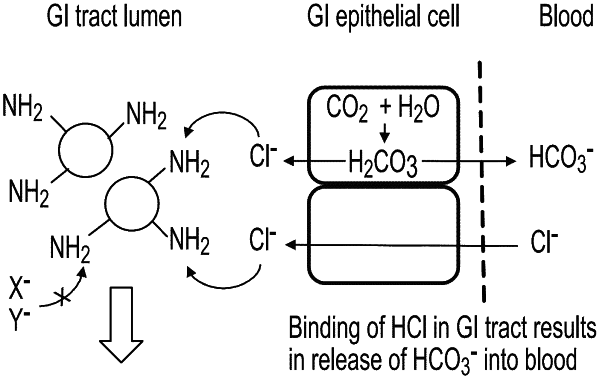
1. A method of treating a patient with chronic kidney disease and eubicarbonatemic metabolic acidosis, wherein the method comprises oral administration of a nonabsorbable pharmaceutical composition comprising a proton-binding, crosslinked amine polymer comprising the residue of an amine corresponding to Formula 1:
 wherein R1, R2 and R3 are independently hydrogen, hydrocarbyl, substituted hydrocarbyl provided, however, at least one of R1, R2 and R3 is other than hydrogen, the crosslinked amine polymer has an equilibrium swelling ratio in deionized water of about 5 or less, and the crosslinked amine polymer binds a molar ratio of chloride ions to interfering ions of at least 0.35:1, respectively, in an interfering ion buffer at 37° C. wherein (i) the interfering ions are phosphate ions and the interfering ion buffer is a buffered solution at pH 5.5 of 36 mM chloride and 20 mM phosphate or (ii) the interfering ions are phosphate, citrate and taurocholate ions (combined amount) and the interfering ion buffer is a buffered solution at pH 6.2 including 36 mM chloride, 7 mM phosphate, 1.5 mM citrate, and 5 mM taurocholate.
|