| CPC A61K 31/4725 (2013.01) [A61K 31/496 (2013.01); A61K 31/5377 (2013.01); A61K 31/5386 (2013.01); C07D 401/14 (2013.01); C07D 413/14 (2013.01); C07D 498/08 (2013.01)] | 20 Claims |
|
1. A method of treating a methylthioadenosine phosphorylase-deficient (MTAP-deficient) and/or a methylthioadenosine-accumulating (MTA-accumulating) cancer selected from the group consisting of glioblastoma, malignant peripheral nerve sheath tumors (MPNST), esophageal cancer, bladder cancer, pancreatic cancer, mesothelioma, melanoma, non-small cell lung cancer, astrocytoma, diffuse large B-cell lymphoma (DLBCL), leukemia, head and neck cancer, myxofibrosarcoma, cholangiosarcoma, cancer of the brain, stomach cancer, kidney cancer, breast cancer, cancer of the endometrium, urinary tract cancer, liver cancer, soft tissue cancer, pleura cancer, large intestine cancer and sarcoma in a subject in need thereof by administering to the subject a therapeutically effective amount of a compound or a pharmaceutically acceptable salt thereof wherein the compound is of formula (I)
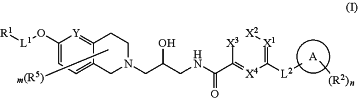 wherein
X1, X2, X3 and X4 are each independently N or CRx;
Y is N, CH or CR5;
L1 is a bond or C1-C4-alkylene substituted with 0-2 instances of R6;
L2 is a bond, —NH— or —O—;
Ring A is a carbocycle, heterocycle, 5-6 member monocyclic heteroaryl or a monocyclic aryl;
R1 is a 3-7 membered carbocycle, a 4-7 membered heterocycle or a 5-6 membered heteroaryl substituted with 0-3 instances of R4;
each R2 is independently selected from ═O, halo, —CN, —C1-C6 alkyl, —C1-C6 heteroalkyl, —C1-C6 haloalkyl, —C3-C9 carbocyclyl, —C3-C9 heterocyclyl, heterocyclylalkyl, cycloalkylalkyl, —OR3, —N(R3)2, —C(═O)R3, —C(═O)OR3, —CH2C(═O)R3, —NR3C(═O)R3, —NR3C(═O)OR3, —C(═O)N(R3)2, —OC(═O)N(R3)2, —S(═O)R3, —S(═O)2R3, —SR3, —S(═O)(═NR3)R3, —NR3S(═O)2R3 and —S(═O)2N(R3)2;
each R3 is independently selected from H, C1-C6 alkyl, C1-C6 heteroalkyl, C3-C7 carbocyclyl, C3-C7 heterocyclyl, cycloalkylalkyl, heterocyclylalkyl, aryl, 5-6 membered heteroaryl, arylalkyl and heteroarylalkyl wherein each alkyl, carbocyclyl, heterocyclyl, cycloalkylalkyl, heterocyclylalkyl, aryl, heteroaryl, arylalkyl and heteroarylalkyl is optionally substituted;
each R4 is independently selected from ═O, halo, —CN, —C1-C6 alkyl, —C1-C6 heteroalkyl, —C1-C6 haloalkyl, —C3-C9 carbocyclyl, —C3-C9 heterocyclyl, heterocyclylalkyl, cycloalkylalkyl, —OR3, —N(R3)2, —C(═O)R3, —C(═O)OR3, —NR3C(═O)R3, —NR3C(═O)OR3, —C(═O)N(R3)2, —OC(═O)N(R3)2, —S(═O)R3, —S(═O)2R3, —SR3, —S(═O)(═NR3)R3, —NRS(═O)2R3 and —S(═O)2N(R3)2;
each Rx is independently selected from hydrogen, halo, —CN, —C1-C6 alkyl, —C1-C6 heteroalkyl, —C1-C6 haloalkyl, —C3-C9 carbocyclyl, —C3-C9 heterocyclyl, heterocyclylalkyl, cycloalkylalkyl, —OR3, —N(R3)2, —C(═O)R3, —C(═O)OR3, —NR3C(═O)R3, —NR3C(═O)OR3, —C(═O)N(R3)2, —OC(═O)N(R3)2, —S(═O)R3, —S(═O)2R3, —SR3, —S(═O)(═NR3)R3, —NRS(═O)2R3 and —S(═O)2N(R3)2 wherein each alkyl, carbocyclyl, heterocyclyl, heterocyclylalkyl, cycloalkylalkyl is optionally substituted;
each R5 is independently selected from ═O, halo, —CN, —C1-C6 alkyl, —C1-C6 heteroalkyl, —C1-C6 haloalkyl, —C3-C9 carbocyclyl, —C3-C9 heterocyclyl, C6-C10 aryl, C5-C10 heteroaryl, cycloalkylalkyl, heterocyclylalkyl, arylalkyl, heteroarylalkyl, —OR3, —N(R3)2, —C(═O)R3, —C(═O)OR3, —NR3C(═O)R3, —NR3C(═O)OR3, —C(═O)N(R3)2, —OC(═O)N(R3)2, —S(═O)R3, —S(═O)2R3, —SR3, —S(═O)(═NR3)R3, —NR3S(═O)2R3, —S(═O)2N(R3)2, or two R5 can be taken together with the atoms to which they are attached to form a —C3-C9 carbocyclyl or a —C3-C9 heterocyclyl;
each R6 is independently selected from halo, —C1-C4 alkyl, —C1-C4 haloalkyl, —OC1-C4 alkyl, —OC1-C4 haloalkyl, or two R6 can be taken together with the atoms to which they are attached to form a C3-C7 carbocycle or a C3-C7 heterocycle;
m is 0, 1, 2 or 3; and
n is 0, 1, 2 or 3.
|