| CPC A61K 31/454 (2013.01) [A61K 9/0053 (2013.01); A61P 13/08 (2018.01); A61P 35/00 (2018.01); A61P 35/04 (2018.01)] | 22 Claims |
|
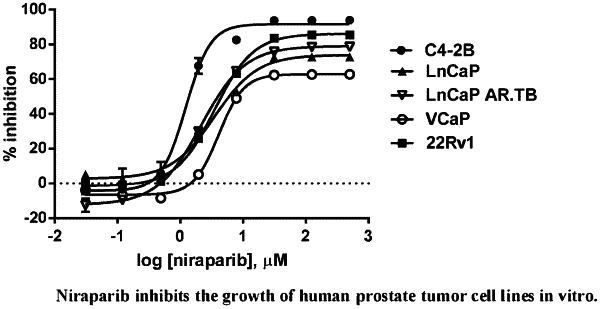
1. A method of treating prostate cancer in a human in need of such treatment comprising administering to the human a pharmaceutically acceptable amount of niraparib or a salt thereof, wherein the prostate cancer is antiandrogen resistant and wherein the human (a) is not BRCA deficient or (b) is carrying at least one DNA repair anomaly in a gene selected from the group consisting of FANCA, PALB2, CHEK2, BRIP1, HDAC2, and ATM.
|