| CPC A61K 31/4375 (2013.01) [A61K 45/06 (2013.01); A61P 35/04 (2018.01)] | 11 Claims |
|
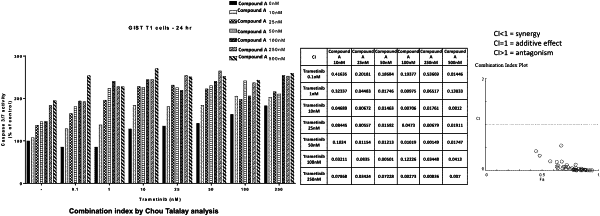
1. A method of treating a tumor having one or more c-KIT mutations in a patient in need thereof, comprising administering to the patient:
an effective amount of 1-[4-bromo-5-[1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl]-2-fluorophenyl1-3-phenylurea, or a pharmaceutically acceptable salt thereof; and
an effective amount of one or more MAPKAP kinase inhibitors selected from the group consisting of trametinib, binimetinib, cobimetinib, and ulixertinib,
wherein the tumor is gastrointestinal stromal tumor (GIST).
|