| CPC A61K 31/198 (2013.01) [A61K 9/5005 (2013.01); A61P 25/16 (2018.01)] | 17 Claims |
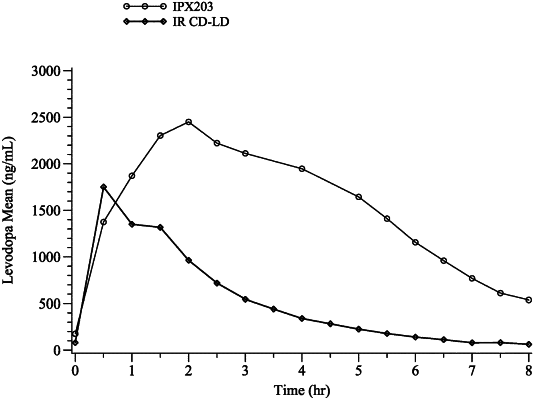
|
1. A method for treating a patient diagnosed with Parkinson's disease comprising:
i) selecting a patient diagnosed with Parkinson's disease and being treated with oral immediate release levodopa tablets administered three, four, five or more times a day for a total daily levodopa dose of 500 mg or less;
ii) determining the amount of levodopa administered to the patient with each administration of the one or more immediate release levodopa tablets of step (i);
iii) discontinuing the administration of the immediate release levodopa tablets; and
iv) orally administering one or more multiparticulate controlled release levodopa dosage forms twice or thrice a day to the patient, wherein the amount of levodopa administered with each administration of the multiparticulate controlled release levodopa dosage form is 2.8 times the amount of levodopa the patient was receiving with each administration of the one or more immediate release levodopa tablets of step (i),
wherein the patient after receiving treatment with the multiparticulate controlled release levodopa dosage form exhibits a decrease of at least 10% of the patient's total post dose “Off” time as compared to post dose of the oral immediate release levodopa tablets
wherein the multiparticulate controlled release dosage form comprises:
(a) a plurality of controlled release components comprising a core comprising levodopa wherein the core is coated with a layer comprising a muco-adhesive polymer and externally coated with a layer comprising an enteric coating polymer; and
(b) an immediate release component comprising levodopa and carbidopa.
|