| CPC G06Q 10/087 (2013.01) [G06F 9/547 (2013.01); G06N 3/08 (2013.01); G06Q 30/0201 (2013.01); G06Q 50/22 (2013.01)] | 30 Claims |
|
1. A method for pharmaceutical ordering and procurement for an ordering location, the method comprising:
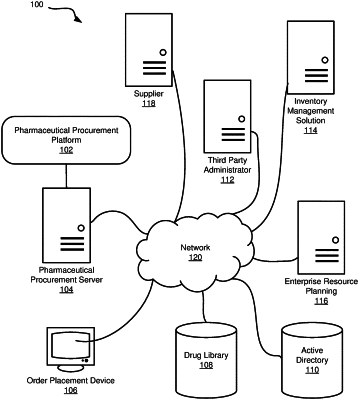
extracting drug product data for a plurality of drug products from a drug library, wherein each of the plurality of drug products is associated with a unique drug product identifier;
parsing the drug product data into one or more tables of a database that is in communication with a network;
extracting inventory data for the ordering location from an inventory management solution that manages drug inventory for the ordering location, wherein the inventory management solution comprises a robotic automated dispenser that dispenses at least a portion of the plurality of drug products at the ordering location;
extracting current drug product pricing information from a plurality of pharmaceutical suppliers, wherein the current drug product pricing information is associated with the plurality of drug products;
parsing the current drug product pricing information into the database;
determining a medication need for procuring one or more of the plurality of drug products for the ordering location at least based on the inventory data, wherein the ordering location comprises one or more of a hospital, a clinic, a surgical center, an urgent care facility, a pharmacy, or a consolidated service center;
identifying drug product preferences for the ordering location, wherein identifying the drug product preferences comprises:
determining a pricing preference for the ordering location;
identifying a formulary applicable to the ordering location, wherein the formulary identifies two or more of the plurality of drug products that are interchangeable, and wherein the formulary further identifies whether the ordering location must comply with a restriction associated with any of the plurality of drug products;
determining whether a governmental pharmaceutical regulation applies to the ordering location; and
identifying medication ordering permissions for the ordering location indicating which of the plurality of drug products the ordering location has permission to purchase or obtain;
querying the one or more tables of the database to identify one or more of the plurality of drug products that satisfy the medication need, wherein each of the one or more of the plurality of drug products is associated with a unique drug product identifier;
selecting a recommended drug product based on the drug product preferences for the ordering location, wherein the recommended drug product is associated with a delivery form, a release mechanism, and a package type that satisfies the medication need;
calculating a recommended quantity of the recommended drug product to be procured for the ordering location based on an expected unit-dose disbursement of the recommended drug product by the ordering location; and
selecting a recommended pharmaceutical supplier for acquiring at least a portion of the recommended quantity of the recommended drug product, wherein the recommended pharmaceutical supplier based at least in part on the current drug product pricing information extracted from the plurality of pharmaceutical suppliers; and
generating a recommended pharmaceutical order for the ordering location, wherein the recommended pharmaceutical order comprises an indication of the recommended drug product, the recommended quantity, the delivery form, the release mechanism, the package type, and the recommended pharmaceutical supplier.
|