| CPC C09B 69/102 (2013.01) [C07C 215/68 (2013.01); C07F 9/091 (2013.01); C07F 9/2408 (2013.01); C09B 69/103 (2013.01); C09B 69/109 (2013.01); G01N 33/582 (2013.01)] | 17 Claims |
|
1. A compound having the following structure (II):
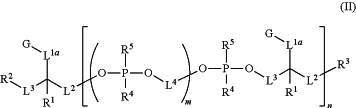 or a stereoisomer, salt or tautomer thereof, wherein:
G is, at each occurrence, independently a moiety comprising an aldehyde, oxime, hydrazone, alkyne, amine, azide, acylazide, acylhalide, nitrile, nitrone, sulfhydryl, disulfide, sulfonyl halide, isothiocyanate, activated ester, ketone, α, β-unsaturated carbonyl, alkene, maleimide, α-haloimide, epoxide, aziridine, tetrazine, tetrazole, phosphine, biotin, or thiirane functional group, wherein the activated ester is an N-succinimide ester, imidoester or polyflourophenyl ester;
L1a, L2 and L3 are, at each occurrence, independently an optional alkylene, alkenylene, alkynylene, heteroalkylene, heteroalkenylene, heteroalkynylene or heteroatomic linker;
L4 is, at each occurrence, independently an alkylene, alkenylene, alkynylene, heteroalkylene, heteroalkenylene or heteroalkynylene linker;
R1 is, at each occurrence, independently H, alkyl or alkoxy;
R2 and R3 are each independently H, OH, SH, alkyl, alkoxy, alkylether, —OP(═Ra)(Rb)Rc, Q, a linker comprising a covalent bond to Q, a linker comprising a covalent bond to an analyte molecule, a linker comprising a covalent bond to a solid support or a linker comprising a covalent bond to a further compound of structure (II), wherein: Ra is O or S; Rb is OH, SH, O−, S−, ORd or SRd; Rc is OH, SH, O−, S−, ORd, SRd, alkyl, alkoxy, alkylether, alkoxyalkylether, phosphate, thiophosphate, phosphoalkyl, thiophosphoalkyl, phosphoalkylether or thiophosphoalkylether; and Rd is a counter ion;
R4 is, at each occurrence, independently OH, SH, O−, S−, ORd or SRd;
R5 is, at each occurrence, independently oxo, thioxo or absent;
Q is, at each occurrence, independently a moiety comprising a sulfhydryl, disulfide, activated ester, isothiocyanate, azide, alkyne, alkene, diene, dienophile, acid halide, sulfonyl halide, phosphine, a-haloamide, biotin, amino or maleimide functional group, wherein the activated ester is an N-succinimide ester, imidoester or polyflourophenyl ester;
m is, at each occurrence, independently an integer of zero or greater, provided that at least one occurrence of m is an integer of one or greater; and
n is an integer of one or greater.
|