| CPC C07D 243/14 (2013.01) [C07D 401/12 (2013.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 413/08 (2013.01); C07D 413/12 (2013.01); C07D 417/12 (2013.01); C07D 471/04 (2013.01); C07D 471/10 (2013.01); C07D 487/04 (2013.01); C07D 498/04 (2013.01); C07D 519/00 (2013.01); C07B 2200/13 (2013.01)] | 27 Claims |
|
1. A compound of formula (I):
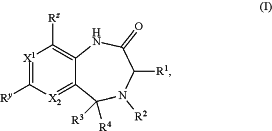 or a pharmaceutically acceptable salt, stereoisomer, or tautomer thereof, wherein:
X1 and X2 are each independently N or C-Rx;
each Rx, Ry, and Rz is independently H, halo, C3-10cycloalkyl, C3-10cycloalkenyl, or C6-20aryl;
R1 is C3-12alkyl, C2-12alkenyl, C2-12alkynyl, C3-10cycloalkyl, C3-10cycloalkenyl, or
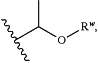 wherein Rw is C1-12alkyl;
R2 is:
a) C(O)—Rh, wherein Rh is
(i) amino optionally substituted with one or more Rg, wherein Rq is C1-12alkyl, C3-10cycloalkyl, C6-20aryl, 3-15 membered heterocyclyl, or 5-20 membered heteroaryl, wherein the C1-12alkyl, C3-10cycloalkyl, C6-20aryl, 3-15 membered heterocyclyl, or 5-20 membered heteroaryl of Rq is optionally substituted with one or more Rp, wherein Rp is OH, cyano, halo, oxo, —C(O)NH2, —C(O)NH(C1-12alkyl), —C(O)-(3-15 membered heterocyclyl), —S(O)—C1-12alkyl, —S(O)2—C1-12alkyl, —S(O)2—NH2, —N(C1-12alkyl)2, —NHC(O)—C1-12alkyl, —NHC(O)—NH2, C6-20aryl, 3-15 membered heterocyclyl, or 5-20 membered heteroaryl, wherein the C6-20aryl, 3-15 membered heterocyclyl, 5-20 membered heteroaryl, or the 3-15 membered heterocyclyl of the C(O)-(3-15 membered heterocyclyl) of Rp is independently optionally substituted with one or more Rv, wherein Rv is OH, oxo, —C(O)NH2, —C(O)OH, or C1-12alkyl, wherein the C1-12alkyl of Rv is further optionally substituted with one or more OH,
C1-3alkoxy,
—C(O)NH2,
C3-10cycloalkyl,
C3-10cycloalkenyl, wherein the C3-10cycloalkenyl is unsubstituted or substituted with one or more oxo,
C6-20aryl, wherein the C6-20aryl is unsubstituted or substituted with one or more OH, 5-20 membered heteroaryl, or —C(O)NH2,
3-15 membered heterocyclyl, wherein the 3-15 membered heterocyclyl is unsubstituted or substituted with one or more Rj, wherein Rj is OH, oxo, halo, NH2, —N(C1-12alkyl)2, —N(C1-12alkyl)-C(O)C1-12alkyl, —NH—SO2—C1-12alkyl, —SO2—C1-12alkyl, C1-12alkyl, C1-12alkoxy, —C(O)OH, —C(O)—C1-12alkoxy, —C(O)NH2, —C(O)NH(C1-12alkyl), —C(O)N(C1-12alkyl)2, 3-15 membered heterocyclyl, or 5-20 membered heteroaryl, wherein the C1-12alkyl, C1-12alkoxy, 3-15 membered heterocyclyl, or 5-20 membered heteroaryl of Rj is independently further optionally substituted with one or more Rk, wherein Rk is OH, C1-12alkyl, —C(O)NH2, —C(O)NH(C1-12alkyl), —C(O)N(C1-12alkyl)2, C6-20aryl, or 5-20 membered heteroaryl, wherein the C1-12alkyl of Rk is independently further optionally substituted with one or more OH, or
5-20 membered heteroaryl, wherein the 5-20 membered heteroaryl is unsubstituted or substituted with one or more Rt, wherein Rt is OH, NH2, C1-12alkyl, —C(O)OH, —C(O)—C1-12alkoxy, —C(O)NH2, —C(O)NH(C1-12alkyl), —C(O)N(C1-12alkyl)2, or —C(O)-(3-15 membered heterocyclyl), wherein the C1-12alkyl of Rt, the 3-15 membered heterocyclyl of the —C(O)-(3-15 membered heterocyclyl) of Rt, the C1-12alkyl of the —C(O)NH(C1-12alkyl) of Rt, or the C1-12alkyl of the —C(O)N(C1-12alkyl)2 of Rt is independently further optionally substituted with one or more OH, —C1-12alkoxy, or —C(O)NH2, or
(ii) C1-12alkyl, wherein the C1-12alkyl is unsubstituted or is substituted with one or more Rn, wherein Rn is OH, oxo, halo, cyano, —C(O)NH2, amino, sulfonyl, C1-12alkoxy, C6-20aryloxy, C3-10cycloalkyl, C3-10cycloalkenyl, 3-15 membered heterocyclyl, or 5-20 membered heteroaryl, or
b) C1-12alkyl, wherein the C1-12alkyl is unsubstituted or is substituted with one or more Rm, wherein
Rm is OH, halo, cyano, oxo, C1-12alkyl, C1-C3 alkoxy, C6-20aryloxy, —C(O)NH2, —C(O)NH(C1-12alkyl), —C(O)N(C1-12alkyl)2, —C(O)OH, —C(O)—C1-12alkoxy, —C(O)-(3-15 membered heterocyclyl), NH2, —NH(C1-12alkyl), —N(C1-12alkyl)2, —NHC(O)—C1-12alkyl, —NHC(O)—NH2, —NH—SO2—C1-12alkyl, —S(O)—C1-12alkyl, —S(O)2—C1-12alkyl, —S(O)2—NH2, C3-10cycloalkyl, or 3-15 membered heterocyclyl, wherein
the C1-12alkyl, C6-20aryloxy, the C1-12alkyl of —C(O)NH(C1-12alkyl), the C1-12alkyl of —C(O)N(C1-12alkyl)2, —C(O)OH, —C(O)—C1-12alkoxy, the 3-15 membered heterocyclyl of —C(O)-(3-15 membered heterocyclyl), NH2, the C1-12alkyl of —NH(C1-12alkyl), the C1-12alkyl of —N(C1-12alkyl)2, the C1-12alkyl of —NHC(O)—C1-12alkyl, —NHC(O)—NH2, the C1-12alkyl of —NH—SO2—C1-12alkyl, the C1-12alkyl of —S(O)—C1-12alkyl, the C1-12alkyl of —S(O)2—C1-12alkyl, —S(O)2—NH2, C3-10cycloalkyl, or 3-15 membered heterocyclyl of Rm is further optionally substituted by one or more OH, halo, cyano, oxo, C1-12alkyl, C1-12alkoxy, —C(O)NH2, —C(O)NH(C1-12alkyl), —C(O)N(C1-12alkyl)2, C(O)OH, NH2, —NH(C1-12alkyl), —N(C1-12alkyl)2, C3-10cycloalkyl, C6-20aryl, 3-15 membered heterocyclyl, or 5-20 membered heteroaryl, or
c) C3-10cycloalkenyl, wherein the C3-10cycloalkenyl is unsubstituted or is substituted with one or more Ri, wherein Ri is oxo, NH2, —NH(C1-12alkyl), —N(C1-12alkyl)2, or 3-15 membered heterocyclyl, wherein the 3-15 membered heterocyclyl of Ri, the C1-12alkyl of the —NH(C1-12alkyl) of Ri, or the C1-12alkyl of the —N(C1-12alkyl)2 of Ri is independently optionally substituted with one or more OH or C1-12alkoxy, or
d) 5-20 membered heteroaryl, wherein the 5-20 membered heteroaryl is unsubstituted or substituted with one or more OH, NH2, C1-12alkyl, or C1-12alkoxy, or
e) 3-15 membered heterocyclyl, wherein the 3-15 membered heterocyclyl is unsubstituted or substituted with one or more OH, oxo, NH2, or C1-12alkyl, or
f) amidinyl, wherein the amidinyl is unsubstituted or substituted with one or more Rs, wherein Rs is OH, cyano, C1-12alkyl, —C(O)—C1-12alkyl, —C(O)—C1-12alkoxy, C6-20aryloxy, or —SO2—C1-12alkyl, or
g) sulfonyl, wherein the sulfonyl is unsubstituted or substituted with one or more Ru, wherein Ru is C1-12alkyl, NH2, —NH(C1-12alkyl), —N(C1-12alkyl)2, or C6-20aryl, wherein the C1-12alkyl or C6-20aryl of Ru is independently further optionally substituted with one or more halo or C1-12alkoxy, or
h) cyano, and
R3 is H, C1-12alkyl, —C(O)NH2, or —C(O)—C1-12alkoxy; or
R2 and R3 are taken together with the atoms to which they are attached to form a 5- or 6-membered heterocyclyl or 5- or 6-membered heteroaryl, wherein the 5- or 6-membered heterocyclyl or 5- or 6-membered heteroaryl independently comprises two or more annular heteroatoms and is independently optionally substituted with one or more oxo or OH; and
R4 is absent or is H, C1-12alkyl, —C(O)NH2, or —C(O)—C1-12alkoxy.
|