| CPC C07C 217/58 (2013.01) [A23L 33/10 (2016.08); A61K 31/05 (2013.01); A61K 31/12 (2013.01); A61K 31/138 (2013.01); A61K 31/166 (2013.01); A61K 31/192 (2013.01); A61K 31/277 (2013.01); A61K 31/336 (2013.01); A61K 31/355 (2013.01); A61K 31/4015 (2013.01); A61K 31/4155 (2013.01); A61K 31/4184 (2013.01); A61K 31/421 (2013.01); A61K 31/428 (2013.01); A61K 31/4535 (2013.01); A61K 31/4709 (2013.01); A61K 31/4745 (2013.01); A61K 31/496 (2013.01); A61K 31/50 (2013.01); A61K 31/5025 (2013.01); A61K 31/506 (2013.01); A61K 31/517 (2013.01); A61K 31/5377 (2013.01); A61K 31/5415 (2013.01); A61K 31/551 (2013.01); A61K 31/565 (2013.01); A61K 39/3955 (2013.01); A61K 47/54 (2017.08); A61K 47/55 (2017.08); A61K 47/6803 (2017.08); A61K 47/6849 (2017.08); A61P 25/28 (2018.01); C07C 43/23 (2013.01); C07C 47/575 (2013.01); C07C 235/06 (2013.01); C07C 237/06 (2013.01); C07C 275/24 (2013.01); C07C 279/12 (2013.01); C07D 303/18 (2013.01); C07B 2200/07 (2013.01)] | 12 Claims |
|
1. A p62 ligand compound of the following Chemical Formula 1 or a pharmaceutically acceptable salt thereof:
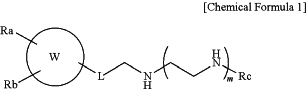 in Chemical Formula 1,
W is C6-C10 aryl;
n1 is an integer of 1 to 4;
m is an integer of 0 to 2;
Ra is R1 or —OR1,
where R1 is (CH2)n2—R′1,
R′1 is phenyl which is unsubstituted or substituted by hydroxy, halogen, C1-4 alkyl′, C1-4 alkoxy, nitro, amino, (C1-4 alkyl)amino, or di(C1-4 alkyl)amino,
n2 is an integer of 1 to 6;
Rb is —OR2,
where R2 is hydrogen or —(CH2)n3—R′2,
R′2 is phenyl which is unsubstituted or substituted by hydroxy, halogen, C1-4 alkyl, C1-4 alkoxy, nitro, amino, (C1-4 alkyl)amino, or di(C1-4 alkyl)amino,
n3 is an integer of 1 to 6;
Rc is —(CH2)n4—OH, —(CH2)n4—NH—C(═NH)NH2, —C(═NH)NH2, C1-6 alkyl, —CH(R3)—COO—R4, or —CH(COO—R4)—CH2CH2CH2—NH—C(═NH)NH2, —(CH2)n4—O—(CH2)n4—OR4, —CONH(CH2)n4—OR4, —CO(CH2)n5—OR4, —(CH2)n5—CH(NH2)—COOR4, —(CH2)n5—CONHR4,
n4 is an integer of 2 to 4,
n5 is an integer of 1 to 4,
R3 is hydrogen or C1-4 alkyl,
R4 is hydrogen, or C1-4 alkyl, and
wherein the p62 ligand compound is optically pure (R) form, and
wherein the compound of Chemical Formula 1 is not a compound selected from the group consisting of the following compounds:
1) (R)-1-(3,4-(bis(benzyloxy)phenoxy)-3-((2-hydroxyethyl)amino)propan-2-ol (YOK-1106);
2) (R)-1-(3,4-(bis((4-chlorobenzyl)oxy)phenoxy)-3-((2-hydroxyethyl)amino)propan-2-ol (YT-6-1);
3) (R)-1-(3-((4-chlorobenzyl)oxy)phenoxy)-3-((2-hydroxyethyl)amino)propan-2-ol (YT-6-2);
4) (R)-1-(3-((4-chlorobenzyl)oxy)phenoxy)-3-((2-hydroxyethyl)amino)propan-2-ol (YT-6-7);
5) (R)-1-(3-((4-fluorobenzyl)oxy)phenoxy)-3-((2-hydroxyethyl)amino)propan-2-ol (YT-6-8);
6) (R)-1-(2-((3-(3,4-bis(benzyloxy)phenoxy)-2-hydroxypropyl)amino)ethyl)guanidine (YOK-1109);
7) (R)-1-(2-((3-(3,4-diphenethoxyphenoxy)-2-hydroxypropyl)amino)ethyl)guanidine (YOK-2209);
8) (R)-1-(2-((3-(3,4-bis(4-chlorobenzyl)oxy)phenoxy)-2-hydroxypropyl)amino)ethyl)guanidine (YT-9-1);
9) (R)-1-(2-((3-(3,4-bis(4-fluorobenzyl)oxy)phenoxy)-2-hydroxypropyl)amino)ethyl)guanidine (YT-9-2);
10) (R)-1-(3-(3,4-bis(benzyloxy)phenoxy)-2-hydroxypropyl)guanidine (YOK-1107);
11) (R)-1-(3-(3,4-diphenethoxyphenoxy)-2-hydroxypropyl)guanidine (YOK-2207);
12) (R)-1-(3,4-bis(benzyloxy)phenoxy)-3-(isopropylamino)propan-2-ol (YOK-1104);
13) (R)-1-(3,4-diphenethoxyphenoxy)-3-(isopropylamino)propan-2-ol (YOK-2204);
14) (R)-1-(3,4-bis(3-phenylpropoxy)phenoxy)-3-(isopropylamino)propan-2-ol (YOK-3304);
15) (R)-1-(3,4-bis(4-phenylbutoxy)phenoxy)-3-(isopropylamino)propan-2-ol (YOK-4404);
16) (R)-1-(4-(benzyloxy)-3-phenethoxyphenoxy)-3-(isopropylamino)propan-2-ol (YOK-1204);
17) (R)-1-(4-(benzyloxy)-3-(3-phenylpropoxy)phenoxy)-3-(isopropylamino)propan-2-ol (YOK-1304);
18) (R)-1-(3,4-bis((4-chlorobenzyl)oxy)phenoxy)-3-(isopropylamino)propan-2-ol (YT-4-1); and
19) (R)-1-(3,4-bis((4-fluorobenzyl)oxy)phenoxy)-3-(isopropylamino)propan-2-ol (YT-4-2).
|