| CPC A61N 1/3718 (2013.01) [A61N 1/086 (2017.08); A61N 1/3706 (2013.01); A61N 1/378 (2013.01); G01R 31/50 (2020.01)] | 17 Claims |
|
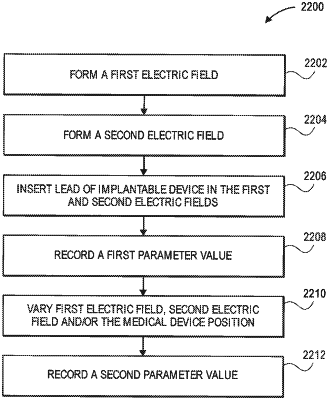
1. A method of validating safety of a medical device in a presence of a magnetic resonance imaging field, the method comprising:
forming a first electric field with a first electric field generating device;
forming a second electric field with a second electric field generating device;
inserting at least a portion of the medical device into the first electric field and into the second electric field at a first position;
recording a first measured parameter value of the lead in the first electric field and the second electric field in the first position;
changing at least one of: the medical device position in at least one of the first electric field or the second electric field, the first electric field, or the second electric field; and
recording a second measured parameter value of the lead in the first electric field and the second electric field after changing the at least one of the medical device position in at least one of the first electric field or the second electric field, the first electric field, or the second electric field;
validating safety of the medical device based on a comparison related to the first measured parameter value and the second measured parameter value.
|