| CPC A61L 27/54 (2013.01) [A61F 2/28 (2013.01); A61K 31/573 (2013.01); A61L 27/06 (2013.01); A61L 27/18 (2013.01); A61F 2002/2825 (2013.01); A61F 2002/3021 (2013.01); A61F 2002/30593 (2013.01); A61F 2002/3085 (2013.01); A61F 2002/30884 (2013.01); A61F 2310/00023 (2013.01); A61L 2430/02 (2013.01); A61L 2430/24 (2013.01)] | 36 Claims |
|
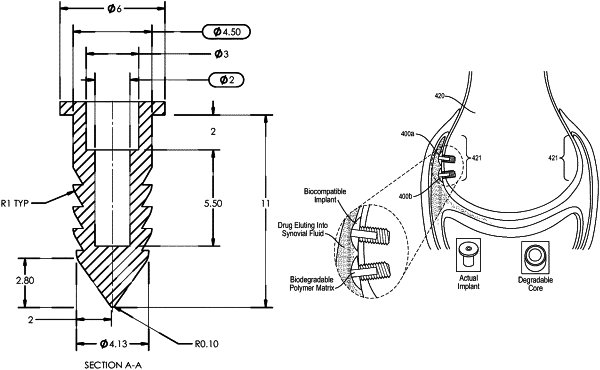
1. An intra-articular implant sized and configured to be implanted in a periarticular region of a joint, the intra-articular implant comprising:
an elongate body extending from a proximal end to a distal end, the elongate body comprising a bone anchor;
a flange disposed at the proximal end and extending radially outwards from the elongate body, the flange configured to be exposed to synovial fluid of the joint;
a bore extending from an opening at the proximal end into the elongate body, wherein the bore comprises an inner surface where it extends into the elongate body;
one or more fixation members disposed on an outer surface of the elongate body; and
a payload disposed within the bore, wherein the payload comprises a therapeutic agent configured to elute constantly and continuously over a predetermined time period,
wherein the payload erodes during elution, wherein a substantially constant surface area of an exposed portion of the payload is present throughout elution, and wherein the inner surface of the bore of the intra-articular implant is sealed and only the opening at the proximal end of the bore exposes the constant surface area of the exposed portion of the payload,
wherein the bore comprises a depth of about 0.5 mm to about 12 mm,
wherein the depth of the bore is larger than a maximum height of the flange,
wherein a length from the proximal end to the distal end is about 7 mm to about 12 mm,
wherein the substantially constant surface area of the exposed portion of the payload is substantially planar with a plane of the flange throughout elution,
wherein, except for the opening at the proximal end, the intra-articular implant is devoid of any means exposing payload to bodily environment,
wherein the intra-articular implant is configured to achieve substantially zero-order kinetic drug delivery in said periarticular region of the joint.
|