| CPC A61K 9/167 (2013.01) [A61K 9/1611 (2013.01); A61K 9/1617 (2013.01); A61K 9/1623 (2013.01); A61K 9/1635 (2013.01); A61K 9/1652 (2013.01); A61K 31/403 (2013.01)] | 29 Claims |
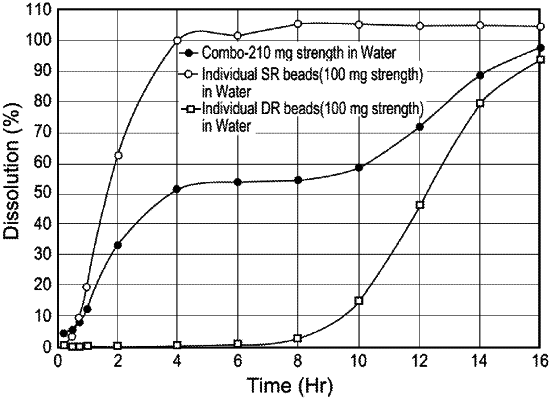
|
1. A pharmaceutical formulation dosage form comprising a plurality of centanafadine beads,
wherein the plurality of centanafadine beads comprise centanafadine or a pharmaceutically acceptable salt thereof and an excipient;
wherein the plurality of centanafadine beads comprises a mixture of a collection of one or more immediate release beads and a collection of one or more sustained release beads,
wherein the centanafadine or a pharmaceutically acceptable salt thereof is present in a greater amount, by weight of the centanafadine or pharmaceutically acceptable salt thereof, in the collection of one or more sustained release beads than in the collection of one or more immediate release beads, and up to a ratio of 1:100 parts by weight of centanafadine or salt thereof, in the collection of one or more immediate release beads to the collection of one or more sustained release beads; and
wherein the dosage form consists of centanafadine or a pharmaceutically acceptable salt thereof as a pharmaceutical active agent.
|