| CPC A61K 31/69 (2013.01) [A61K 31/407 (2013.01); A61K 31/427 (2013.01); A61K 31/431 (2013.01); A61K 31/546 (2013.01); A61P 31/04 (2018.01)] | 7 Claims |
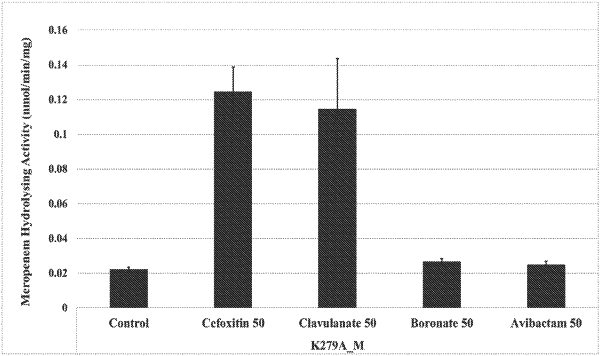
|
1. A combination therapeutic product comprising a β-lactamase inhibitor of Formula Ic, or a pharmaceutically acceptable salt thereof, and one or more β-lactam antibiotics, wherein the β-lactamase inhibitor of Formula Ic has the structural formula shown below:
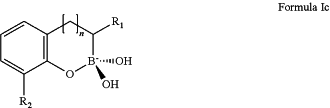 wherein:
n is 0 or 1;
R1 is a substituent group of the formula:
—X—Z
wherein
X is —N(RA3)—C(O), wherein RA3 is selected from hydrogen or (1-2C)alkyl; and
Z is (1-6C)alkyl, aryl or (3-6C)cycloalkyl;
and wherein Z is optionally further substituted by one or more substituent groups independently selected from oxo, halo, cyano, nitro, hydroxy, carboxy, NRA6RA7, —(CH2)p—NRA6RA7 (wherein p is selected from 1 or 2 (1-4C)alkoxy, (1-4C)alkyl or (1-4C)alkanoyl; wherein RA6 and RA7 are each independently selected from hydrogen, (1-4C)alkyl or (1-4C)alkylamino; and
R2 is a substituent of the formula —C(O)OR2A, wherein R2A is selected from hydrogen, (1-6C)alkyl or (3-8C)cycloalkyl.
|