| CPC H01M 4/485 (2013.01) [C01G 23/04 (2013.01); C01G 33/00 (2013.01); C01G 33/006 (2013.01); C01G 37/14 (2013.01); C01G 49/0063 (2013.01); H01M 4/0471 (2013.01); H01M 4/049 (2013.01); H01M 4/131 (2013.01); H01M 4/364 (2013.01); H01M 4/366 (2013.01); H01M 4/583 (2013.01); H01M 4/625 (2013.01); H01M 10/0525 (2013.01); H01M 10/054 (2013.01); C01P 2002/52 (2013.01); C01P 2002/70 (2013.01); C01P 2002/72 (2013.01); C01P 2004/03 (2013.01); C01P 2004/61 (2013.01); C01P 2004/62 (2013.01); C01P 2004/80 (2013.01); C01P 2006/12 (2013.01); C01P 2006/40 (2013.01); H01M 2004/021 (2013.01); H01M 2004/027 (2013.01)] | 24 Claims |
|
1. An active electrode material comprising a mixed niobium oxide, wherein the mixed niobium oxide has the composition M1aM22-aM3bNb34-bO87-c-dQd, wherein: M1 and M2 are different;
M1 is selected from Mg, Ca, Sr, Y, La, Ce, Zr, Hf, V, Nb, Ta, Cr, Mo, W, Mn, Fe, Co, Ni, Cu, Zn, Cd, B, Al, Ga, In, Si, Ge, Sn, Pb, P, Sb, Bi and mixtures thereof;
M2 is Zn or Cu;
M3 is selected from Mg, Ca, Sr, Y, La, Ce, Ti, Zr, Hf, V, Ta, Cr, Mo, W, Mn, Fe, Co, Ni, Cu, Zn, Cd, B, Al, Ga, In, Si, Ge, Sn, Pb, P, Sb, Bi, and mixtures thereof;
Q is selected from F, Cl, Br, I, N, S, Se, and mixtures thereof;
0≤a<1.0; ≤0b≤3.4; −0.5≤c≤4.35; 0≤d≤4.35;
one or more of a, b, c, and d does not equal 0; and
when a, b, and d equal zero, c is greater than zero;
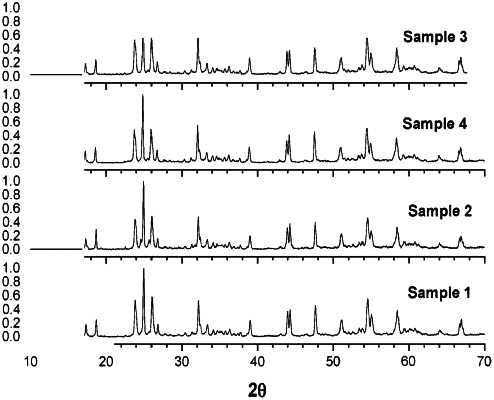
wherein a crystal structure of the mixed niobium oxide as determined by X-ray diffraction corresponds to the crystal structure of one or more of Zn2Nb34O87 and Cu2Nb34O87.
|