| CPC G16H 10/20 (2018.01) [G16H 10/60 (2018.01); G16H 20/10 (2018.01); G16H 20/13 (2018.01); G16H 70/20 (2018.01); G06Q 50/22 (2013.01)] | 20 Claims |
|
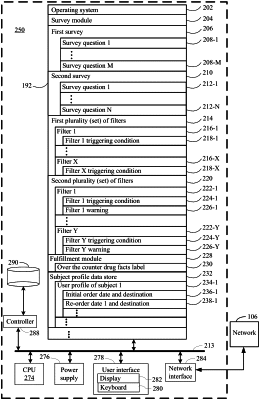
1. A computer system for qualifying a human subject for delivery of a statin pharmaceutical composition over the counter to lower cholesterol, the computer system comprising one or more processors and a memory, the memory comprising non-transitory instructions which, when executed by the one or more processor, perform a method comprising:
a) conducting a first survey thereby obtaining a first plurality of survey results from the subject, wherein the first plurality of survey results comprises:
a gender of the subject,
when the subject is female, whether the subject is (i) pregnant, (ii) breastfeeding, or (iii) planning to become pregnant,
whether the subject has or has ever had a liver disease,
an age of the subject,
a total cholesterol level of the subject,
an HDL cholesterol count of the subject,
a systolic blood pressure of the subject,
a race of the subject,
whether the subject is taking a high blood pressure medication,
whether the subject is taking one or more medications that interact with the statin pharmaceutical composition, wherein the one or more medications include cyclosporine,
a smoking status of the subject,
a diabetes status of the subject,
an alcohol consumption status of the subject,
whether the subject has had an adverse reaction to a cholesterol lowering medication, and
whether the subject has ever had an atherosclerotic cardiovascular event or had a heart procedure; and
b) applying a process to the first plurality of survey results, wherein the process comprises:
i) running all or a portion of the first plurality of survey results against a plurality of filters, wherein, when a respective filter in the plurality of filters is fired, the process is terminated, or the subject is provided with a warning corresponding to the respective filter, and wherein the plurality of filters comprises:
a pregnancy filter,
a cyclosporine filter,
a liver disease or allergic reaction to the statin pharmaceutical composition filter,
a total cholesterol level filter,
a pooled cohort equation filter that incorporates the gender of the subject, the race of the subject, the age of the subject, the total cholesterol level of the subject, the HDL cholesterol count of the subject, the systolic blood pressure of the subject, whether the subject is taking a medication that interacts with a statin, the smoking status of the subject, and the diabetes status of the subject to derive a risk for the atherosclerotic cardiovascular disease, wherein when the risk satisfies a first threshold range or a first threshold value the pooled cohort equation filter is deemed fired,
an age filter,
a drug interaction filter,
an alcohol consumption filter,
an adverse reaction filter, and
an atherosclerotic cardiovascular event filter;
ii) obtaining, when the process is not terminated, acknowledgment from the subject for each warning issued to the subject by any filter in the plurality of filters; and
iii) proceeding with the process when 1) the process is not already terminated by the firing of a filter in the plurality of filters and 2) the subject has acknowledged each warning associated with each filter in the plurality of filters that was fired and that is associated with a warning, wherein the process further comprises:
storing an indication in a subject profile of an initial order for the statin pharmaceutical composition,
communicating an over the counter drug facts label for the statin pharmaceutical composition to the subject, and
authorizing, upon confirmation from the subject that the over the counter drug facts label has been received and read, a provision of the statin pharmaceutical composition to the subject,
wherein the statin pharmaceutical composition is administered to the subject after the authorizing to lower cholesterol.
|