| CPC C12Q 1/6848 (2013.01) [C07D 249/06 (2013.01); C07D 401/14 (2013.01); C07D 403/14 (2013.01); C07D 471/04 (2013.01); C12Q 1/6869 (2013.01)] | 40 Claims |
|
1. A method of enhancing a nucleic acid polymerase reaction, the method comprising:
a. forming a nucleic acid polymerase reaction composition comprising:
i. a template nucleic acid,
ii. a nucleic acid polymerase,
iii. a mixture of nucleotides or nucleotide analogs, and
iv. at least one compound of formula (I); and
b. incubating the nucleic acid polymerase reaction composition under conditions allowing a nucleic acid polymerization reaction, wherein the at least one compound of formula (I) increases the processivity, rate, or fidelity of the nucleic acid polymerase reaction; wherein the compound of formula (I) is represented by:
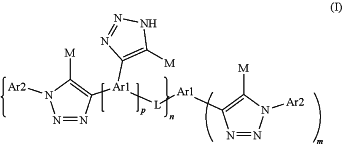 wherein, independently at each occurrence:
m is 1, 2 or 3;
n is 0, 1 or 2;
p is 0, 1 or 2;
Ar1 is optionally substituted aryl;
Ar2 is selected from 5- and 6-membered monocyclic aromatic rings and 9- and 10-membered fused bicyclic rings comprising two 5- and/or 6-membered monocyclic rings fused together, where at least one of the two monocyclic rings is an aromatic ring, where
Ar2 is optionally substituted with one or more substituents selected from halide, C1-C6alkyl, C1-C6haloalkyl, E-CO2R0, E-CONH2, E-CHO, E-C(O)NH(OH), E-N(R0)2, and E-OR0, where
E is selected from a direct bond and C1-C6alkylene; and
R0 is selected from H, C1-C6alkyl and C1-C6haloalkyl,
M is selected from hydrogen, halogen and C1-C4alkyl; and
L is a linking group;
or a solvate, hydrate, tautomer, chelate or salt thereof.
|