| CPC C07H 17/04 (2013.01) [A61P 13/12 (2018.01)] | 23 Claims |
|
1. A compound of Formula (I)
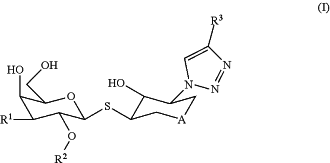 or a pharmaceutically acceptable salt thereof, wherein:
A is O, NH or N(COCH3);
R1 is (R4)CONH— or
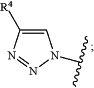 R2 is alkyl, (CO2(R5))alkyl, or (CON(R6)(R7))alkyl;
R3 is cycloalkyl, tetrahydropyranyl, or Ar1, and is substituted with 0-3 substituents selected from cyano, halo, alkyl, haloalkyl, hydroxy, alkoxy, haloalkoxy, CO2R5, and (R6)(R7)N;
R4 is alkyl, cycloalkyl, bicyclo[2.2.1-2]alkyl, or Ar2, and is substituted with 0-5 substituents selected from cyano, halo, alkyl, haloalkyl, hydroxy, alkoxy, haloalkoxy, and CO2R5;
R5 is hydrogen or alkyl;
R6 is hydrogen, alkyl, alkylcarbonyl, or phenyl;
R7 is hydrogen or alkyl;
or (R6)(R7)N taken together is azetidinyl, pyrrolidinyl, piperidinyl, piperazinyl, morpholinyl, or thiomorpholinyl dioxide, and is substituted with 0-3 substituents selected from halo, alkyl, hydroxy, and alkoxy;
Ar1 is phenyl, thiazolyl, pyridinyl, pyridazinyl, pyrimidinyl, or pyrazinyl; and
Ar2 is phenyl, biphenyl, naphthalenyl, thiazolyl, pyridinyl, pyridazinyl, pyrimidinyl, pyrazinyl, quinolinyl, isoquinolinyl, or quinoxalinyl.
|