| CPC C07D 487/04 (2013.01) [A61P 35/02 (2018.01); A61P 35/04 (2018.01); C07B 2200/13 (2013.01)] | 28 Claims |
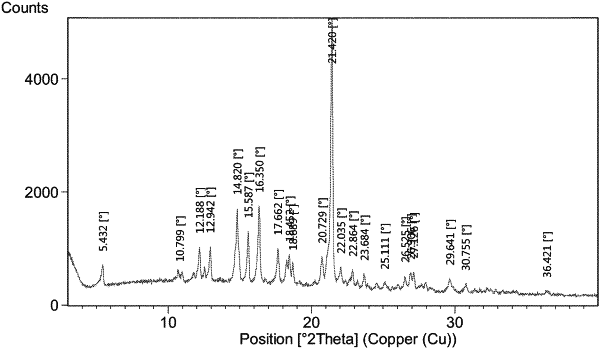
|
1. A method for treating a B-cell proliferative disease in a subject, comprising administering to the subject in need thereof Compound 1,
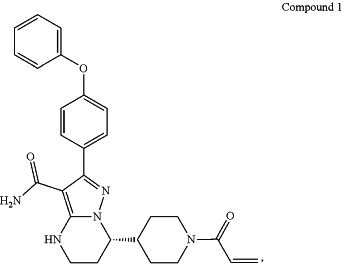 wherein the B-cell proliferative disease is selected from a group consisting of chronic lymphocytic leukemia, small lymphocytic lymphoma, mantle cell lymphoma, Waldenstrom's macroglobulinemia, marginal zone lymphoma, and follicular lymphoma; and
Compound 1 is administrated at a dose of 320 mg once a day (QD).
|