| CPC C07D 413/12 (2013.01) [A61P 35/00 (2018.01); C07D 231/38 (2013.01); C07D 401/04 (2013.01); C07D 401/12 (2013.01); C07D 403/12 (2013.01); C07D 413/14 (2013.01); C07D 417/12 (2013.01); C07D 417/14 (2013.01)] | 24 Claims |
|
1. A compound of the formula:
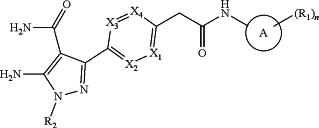 wherein
A is C6-C10 aryl or C5-C6 heteroaryl;
each R1 is independently hydrogen, halogen, C1-C6 alkyl, C1-C6 heteroalkyl, —(C1-C4 alkyl)(C5-C6 heteroalkyl), —(C0-C4 alkyl)(C3-C7 cycloalkyl), —(C0-C4 heteroalkyl)(C3-C7 cycloalkyl), —(C0-C4 alkyl)(C3-C7 cycloheteroalkyl), —(C0-C4 heteroalkyl)(C3-C7 cycloheteroalkyl), —(C0-C4 alkyl)(C4-C10 bicyclyl), —(C0-C4 alkyl)(C5-C6 aryl), —(C0-C4 alkyl)(C5-C6 heteroaryl), —(C0-C4 alkyl)(C4-C10 heterobicyclyl), C5-C12 spiranyl, C5-C12 heterospiranyl, dimethylphosphoryl, adamantyl, C1-C6 alkoxy, —(C1-C4 alkyl)-SO2—(C1-C4 alkyl), difluoromethylsulfanyl, or pentafluorosulfanyl, wherein each R1 is unsubstituted or when it is capable of being substituted, it is substituted with one or more substituents that are independently selected from the group consisting of halogen, cyano, hydroxyl, oxo, methyl, methoxy, hydroxymethyl, ethyl, ethoxy, hydroxyethyl, methylamine, N,N-dimethylmethylamine, mono-, di, or trihalomethoxy, mono-, di-, or tri-halomethyl, and C0-C3 alkyl-pyrrolidinyl, where the pyrrolidinyl group is unsubstituted or substituted with one, two or 3 independently selected halogen atoms, and wherein two R1 groups can fuse to form a ring structure that includes a portion of A and is optionally aromatic, and n is 1, 2, 3, 4, 5, or 6;
X1, X2, X3, and X4 are each independently N, CH, C—CH3, C—CH2—OH, C—OCH3, C—CH2—OCH3, or C-halogen; wherein at least one of X1, X2, X3, and X4 is N, and
R2 is C1-C6 alkyl, —(C0-C4 alkyl)(C3-C7 cycloalkyl), —(C0-C4 alkyl)(C4-C7 heterocycloalkyl), —(C0-C4 alkyl)(C4-C10 bicyclic) each optionally substituted with one or more of halogen, cyano, hydroxyl, oxo, methyl, methoxy, hydroxymethyl, ethyl, ethoxy, hydroxyethyl, cyclopropyl, or mono-, di-, or tri-halomethyl;
or a pharmaceutically acceptable salt thereof.
|