| CPC C07D 409/14 (2013.01) [A61P 1/16 (2018.01); C07D 209/18 (2013.01); C07D 401/04 (2013.01); C07D 403/04 (2013.01); C07D 403/10 (2013.01); C07D 405/04 (2013.01); C07D 405/10 (2013.01); C07D 405/14 (2013.01); C07D 409/04 (2013.01); C07D 413/04 (2013.01); C07D 417/04 (2013.01); C07D 417/14 (2013.01); C07D 451/02 (2013.01); C07D 471/04 (2013.01); C07D 495/04 (2013.01); C07B 2200/05 (2013.01)] | 24 Claims |
|
1. A method for the prophylaxis and/or treatment of diseases amenable for treatment with LXR modulators, comprising administering to a subject in need thereof a therapeutically effective amount of a compound represented by Formula (I)
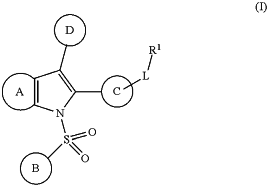 a glycine conjugate, tauro conjugate, enantiomer, diastereomer, tautomer, N-oxide, solvate, prodrug or pharmaceutically acceptable salt thereof,
wherein
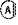 is an annelated 5- to 6-membered cycle forming a 6-membered aryl or a 5- to 6-membered heteroaryl containing 1 to 3 heteroatoms independently selected from N, O and S, wherein this cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from the group consisting of halogen, CN, SF5, NO2, C1-6-alkyl, oxo, C0-6-alkylene-OR11, C0-6-alkylene-(3- to 6-membered cycloalkyl), C0-6-alkylene-(3- to 6-membered heterocycloalkyl), C0-6-alkylene-S(O)nR11, C0-6-alkylene-NR11S(O)2R11, C0-6-alkylene-S(O)2NR11R12, C0-6-alkylene-NR11S(O)2NR11R12, C0-6-alkylene-CO2R11, O—C1-6-alkylene-CO2R11, C0-6-alkylene-O—COR11, C0-6-alkylene-CONR11R12, C0-6-alkylene-NR11—COR11, C0-6-alkylene-NR11—CONR11R12, C0-6-alkylene-O—CONR11R12, C0-6-alkylene-NR11—CO2R11 and C0-6-alkylene-NR11R12, is an annelated 5- to 6-membered cycle forming a 6-membered aryl or a 5- to 6-membered heteroaryl containing 1 to 3 heteroatoms independently selected from N, O and S, wherein this cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from the group consisting of halogen, CN, SF5, NO2, C1-6-alkyl, oxo, C0-6-alkylene-OR11, C0-6-alkylene-(3- to 6-membered cycloalkyl), C0-6-alkylene-(3- to 6-membered heterocycloalkyl), C0-6-alkylene-S(O)nR11, C0-6-alkylene-NR11S(O)2R11, C0-6-alkylene-S(O)2NR11R12, C0-6-alkylene-NR11S(O)2NR11R12, C0-6-alkylene-CO2R11, O—C1-6-alkylene-CO2R11, C0-6-alkylene-O—COR11, C0-6-alkylene-CONR11R12, C0-6-alkylene-NR11—COR11, C0-6-alkylene-NR11—CONR11R12, C0-6-alkylene-O—CONR11R12, C0-6-alkylene-NR11—CO2R11 and C0-6-alkylene-NR11R12,wherein alkyl, alkylene, cycloalkyl and heterocycloalkyl is unsubstituted or substituted with 1 to 6 substituents independently selected from halogen, CN, oxo, hydroxy, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl; and
wherein optionally two adjacent substituents on the aryl or heteroaryl moiety form a 5- to 8-membered partially unsaturated cycle optionally containing 1 to 3 heteroatoms independently selected from O, S or N, and
wherein the new formed cycle is unsubstituted or substituted with 1 to 3 substituents independently selected from halogen, CN, C1-4-alkyl, halo-C1-4-alkyl, 3- to 6-membered cycloalkyl, halo-(3- to 6-membered cycloalkyl), 3- to 6-membered heterocycloalkyl, halo-(3- to 6-membered heterocycloalkyl), OH, oxo, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, O—C1-4-alkyl and O-halo-C1-4-alkyl;
 is selected from the group consisting of 3- to 10-membered cycloalkyl, 3- to 10-membered heterocycloalkyl containing 1 to 3 heteroatoms independently selected from N, O and S, 6- to 14-membered aryl and 5- to 14-membered heteroaryl containing 1 to 4 heteroatoms independently selected from N, O and S, is selected from the group consisting of 3- to 10-membered cycloalkyl, 3- to 10-membered heterocycloalkyl containing 1 to 3 heteroatoms independently selected from N, O and S, 6- to 14-membered aryl and 5- to 14-membered heteroaryl containing 1 to 4 heteroatoms independently selected from N, O and S,wherein cycloalkyl, heterocycloalkyl, aryl and heteroaryl are unsubstituted or substituted with 1 to 6 substituents independently selected from the group consisting of halogen, CN, SF5, NO2, oxo, C1-4-alkyl, C0-6-alkylene-OR21, C0-6-alkylene-(3- to 6-membered cycloalkyl), C0-6-alkylene-(3- to 6-membered heterocycloalkyl), C0-6-alkylene-S(O)nR21, C0-6-alkylene-NR21S(O)2R21, C0-6-alkylene-S(O)2NR21R22, C0-6-alkylene-NR21S(O)2NR21R22, C0-6-alkylene-CO2R21, O—C1-6-alkylene-CO2R21, C0-6-alkylene-O—COR21, C0-6-alkylene-CONR21R22, C0-6-alkylene-NR21—COR21, C0-6-alkylene-NR21—CONR21R22, C0-6-alkylene-O—CONR21R22, C0-6-alkylene-NR21-CO2R21 and C0-6-alkylene-NR21R22,
wherein alkyl, alkylene, cycloalkyl and heterocycloalkyl is unsubstituted or substituted with 1 to 6 substituents independently selected from halogen, CN, oxo, hydroxy, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl,
and wherein optionally two adjacent substituents on the aryl or heteroaryl moiety form a 5- to 8-membered partially unsaturated cycle optionally containing 1 to 3 heteroatoms independently selected from O, S and N, and
wherein this additional cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from halogen, CN, oxo, OH, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl,
and wherein optionally two adjacent substituents on the cycloalkyl or heterocycloalkyl moiety form a 5- to 6-membered unsaturated cycle optionally containing 1 to 3 heteroatoms independently selected from O, S and N,
wherein this additional cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from halogen, CN, oxo, OH, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
 is selected from the group consisting of 6- or 10-membered aryl and 5- to 10-membered heteroaryl containing 1 to 3 heteroatoms independently selected from N, O and S, is selected from the group consisting of 6- or 10-membered aryl and 5- to 10-membered heteroaryl containing 1 to 3 heteroatoms independently selected from N, O and S,wherein aryl and heteroaryl are unsubstituted or substituted with 1 to 4 substituents independently selected from the group consisting of halogen, CN, SF5, NO2, oxo, C1-4-alkyl, C0-6-alkylene-OR31, C0-6-alkylene-(3- to 6-membered cycloalkyl), C0-6-alkylene-(3- to 6-membered heterocycloalkyl), C0-6-alkylene-(6-membered aryl), C0-6-alkylene-(5- to 6-membered heteroaryl), C0-6-alkylene-S(O)nR31, C0-6-alkylene-NR31S(O)2R31, C0-6-alkylene-S(O)2NR31R32, C0-6-alkylene-NR31S(O)2NR31R32, C0-6-alkylene-CO2R31, O—C1-6-alkylene-CO2R31, C0-6-alkylene-O—COR31, C0-6-alkylene-CONR31R32, C0-6-alkylene-NR31—COR31, C0-6-alkylene-NR31—CONR31R32, C0-6-alkylene-O—CONR31R32, C0-6-alkylene-NR31—CO2R31 and C0-6-alkylene-NR31R32,
wherein alkyl, alkylene, cycloalkyl, heterocycloalkyl, aryl and heteroaryl is unsubstituted or substituted with 1 to 6 substituents independently selected from halogen, CN, oxo, hydroxy, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
and wherein optionally two adjacent substituents on the aryl or heteroaryl moiety form a 5- to 8-membered partially unsaturated cycle optionally containing 1 to 3 heteroatoms independently selected from O, S and N, and
wherein this additional cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from halogen, CN, oxo, OH, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
 is selected from the group consisting of 3- to 10-membered cycloalkyl, 3- to 10-membered heterocycloalkyl containing 1 to 3 heteroatoms independently selected from N, O and S, 6- to 14-membered aryl and 5- to 14-membered heteroaryl containing 1 to 4 heteroatoms independently selected from N, O and S, is selected from the group consisting of 3- to 10-membered cycloalkyl, 3- to 10-membered heterocycloalkyl containing 1 to 3 heteroatoms independently selected from N, O and S, 6- to 14-membered aryl and 5- to 14-membered heteroaryl containing 1 to 4 heteroatoms independently selected from N, O and S,wherein cycloalkyl, heterocycloalkyl, aryl and heteroaryl are unsubstituted or substituted with 1 to 6 substituents independently selected from the group consisting of halogen, CN, SF5, NO2, oxo, C1-4-alkyl, C0-6-alkylene-OR21, C0-6-alkylene-(3- to 6-membered cycloalkyl), C0-6-alkylene-(3- to 6-membered heterocycloalkyl), C0-6-alkylene-S(O)nR21, C0-6-alkylene-NR21S(O)2R21, C0-6-alkylene-S(O)2NR21R22, C0-6-alkylene-NR21S(O)2NR21R22, C0-6-alkylene-CR41(═N—OR41), C0-6-alkylene-CO2R21, O—C1-6-alkylene-CO2R21, C0-6-alkylene-O—COR21, C0-6-alkylene-CONR21R22, C0-6-alkylene-NR21—COR21, C0-6-alkylene-NR21—CONR21R22, C0-6-alkylene-O—CONR21R22, C0-6-alkylene-NR21—CO2R21 and C0-6-alkylene-NR21R22,
wherein alkyl, alkylene, cycloalkyl and heterocycloalkyl is unsubstituted or substituted with 1 to 6 substituents independently selected from halogen, CN, oxo, hydroxy, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, CO—OC1-4-alkyl, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
and wherein optionally two adjacent substituents on the aryl or heteroaryl moiety form a 5- to 8-membered partially unsaturated cycle optionally containing 1 to 3 heteroatoms independently selected from O, S and N, and
wherein this additional cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from halogen, CN, oxo, OH, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
and wherein optionally two adjacent substituents on the cycloalkyl or heterocycloalkyl moiety form a 5- to 6-membered unsaturated cycle optionally containing 1 to 3 heteroatoms independently selected from O, S and N,
wherein this additional cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from halogen, CN, oxo, OH, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
wherein
 has a substituent from above in 1,2-orientation regarding to the connection towards has a substituent from above in 1,2-orientation regarding to the connection towards or has an annelated additional cycle in 1,2-orientation;
L is selected from the group consisting of a bond, C1-6-alkylene, C2-6-alkenylene, C2-6-alkinylene, 3- to 10-membered cycloalkylene, 3- to 10-membered heterocycloalkylene containing 1 to 4 heteroatoms independently selected from N, O and S, 6- or 10-membered arylene and 5- to 10-membered heteroarylene containing 1 to 4 heteroatoms independently selected from N, O and S,
wherein alkylene, alkenylene, alkinylene, cycloalkylene, heterocycloalkylene, arylene and heteroarylene are unsubstituted or substituted with 1 to 6 substituents independently selected from the group consisting of halogen, CN, SF5, NO2, oxo, C1-4-alkyl, C0-6-alkylene-OR41, C0-6-alkylene-(3- to 6-membered cycloalkyl), C0-6-alkylene-(3- to 6-membered heterocycloalkyl), C0-6-alkylene-S(O)nR41, C0-6-alkylene-NR41S(O)2R41, C0-6-alkylene-S(O)2NR41R42, C0-6-alkylene-NR41S(O)2NR41R42, C0-6-alkylene-CO2R41, O—C1-6-alkylene-CO2R41, C0-6-alkylene-O—COR41, C0-6-alkylene-CONR41R42, C0-6-alkylene-NR41—COR41, C0-6-alkylene-NR41—CONR41R42, C0-6-alkylene-O—CONR41R42, C0-6-alkylene-NR41—CO2R41 and C0-6-alkylene-NR41R42,
wherein alkyl, alkylene, cycloalkyl and heterocycloalkyl is unsubstituted or substituted with 1 to 6 substituents independently selected from halogen, CN, oxo, hydroxy, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
and wherein optionally two adjacent substituents on the arylene and heteroarylene moiety form a 5- to 8-membered partially unsaturated cycle optionally containing 1 to 3 heteroatoms independently selected from O, S and N, and
wherein this additional cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from halogen, CN, oxo, OH, CO2H, CO2—C1-4-alkyl, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
R1 is selected from the group consisting of H, halogen, CN, SF5, NO2, oxo, C1-4-alkyl, C0-6-alkylene-OR41, Y—C0-6-alkylene-(3- to 6-membered cycloalkyl), Y—C0-6-alkylene-(3- to 6-membered heterocycloalkyl), Y—C0-6-alkylene-(6-membered aryl), Y—C0-6-alkylene-(5- to 6-membered heteroaryl), C0-6-alkylene-S(═O)(—R41)═N—R75, X—C1-6-alkylene-S(═O)(—R41)═N—R75, C0-6-alkylene-S(O)nR41, X—C1-6-alkylene-S(O)nR41, C0-6-alkylene-S(═NR71)R41, X—C1-6-alkylene-S(═NR71)R41, C0-6-alkylene-S(O)(═NR71)R41, X—C1-6-alkylene-S(O)(═NR71)R41, C0-6-alkylene-S(═NR71)2R41, X—C1-6-alkylene-S(═NR71)2R41, C0-6-alkylene-NR41S(O)2R41, X—C1-6-alkylene-NR41S(O)2R41, C0-6-alkylene-S(O)2NR41R42, X—C1-6-alkylene-S(O)2NR41R42, C0-6-alkylene-NR41S(O)2NR41R42, X—C1-6-alkylene-NR41S(O)2NR41R42, C0-6-alkylene-SO3R41, X—C1-6-alkylene-SO3R41, C0-6-alkylene-CO2R41, X—C1-6-alkylene-CO2R41, C0-6-alkylene-O—COR41, X—C1-6-alkylene-O—COR41, C0-6-alkylene-CONR41R42, X—C1-6-alkylene-CONR41R42, C0-6-alkylene-CONR41OR41, X—C1-6-alkylene-CONR41OR41, C0-6-alkylene-CONR41SO2R41, X—C1-6-alkylene-CONR41SO2R41, C0-6-alkylene-NR41—COR41, X—C1-6—C0-6-alkylene-NR41—COR41, C0-6-alkylene-NR41—CONR41R42, X—C1-6-alkylene-NR41—CONR41R42, C0-6-alkylene-O—CONR41R42, X—C1-6-alkylene-O—CONR41R42, C0-6-alkylene-NR41—CO2R41, X—C1-6-alkylene-NR41—CO2R41, C0-6-alkylene-NR41R42, and X—C1-6-alkylene-NR41R42,
wherein alkyl, alkylene, cycloalkyl, heterocycloalkyl, aryl and heteroaryl is unsubstituted or substituted with 1 to 6 substituents independently selected from halogen, CN, oxo, hydroxy, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
and wherein optionally two adjacent substituents on the aryl and heteroaryl moiety form a 5- to 8-membered partially unsaturated cycle optionally containing 1 to 3 heteroatoms independently selected from O, S and N, and
wherein this additional cycle is unsubstituted or substituted with 1 to 4 substituents independently selected from halogen, CN, oxo, OH, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, C1-4-alkyl, halo-C1-4-alkyl, O—C1-4-alkyl and O-halo-C1-4-alkyl;
R11, R12, R21, R22, R31, R32, R41, R42, R51 are independently selected from H and C1-4-alkyl,
wherein alkyl is unsubstituted or substituted with 1 to 3 substituents independently selected from halogen, CN, C1-4-alkyl, halo-C1-4-alkyl, 3- to 6-membered cycloalkyl, halo-(3- to 6-membered cycloalkyl), 3- to 6-membered heterocycloalkyl, halo-(3- to 6-membered heterocycloalkyl), OH, oxo, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, SO3H, O—C1-4-alkyl and O-halo-C1-4-alkyl;
or R11 and R12, R21 and R22, R31 and R32, R41 and R42, respectively, when taken together with the nitrogen to which they are attached complete a 3- to 6-membered ring containing carbon atoms and optionally containing 1 or 2 heteroatoms independently selected from O, S and N; and
wherein the new formed cycle is unsubstituted or substituted with 1 to 3 substituents independently selected from halogen, CN, C1-4-alkyl, halo-C1-4-alkyl, 3- to 6-membered cycloalkyl, halo-(3- to 6-membered cycloalkyl), 3- to 6-membered heterocycloalkyl, halo-(3- to 6-membered heterocycloalkyl), OH, oxo, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, SO3H, O—C1-4-alkyl and O-halo-C1-4-alkyl;
R71 is independently selected from H, CN; NO2, C1-4-alkyl and C(O)—OC1-4-alkyl,
wherein alkyl is unsubstituted or substituted with 1 to 3 substituents independently selected from halogen, CN, C1-4-alkyl, halo-C1-4-alkyl, 3- to 6-membered cycloalkyl, halo-(3- to 6-membered cycloalkyl), 3- to 6-membered heterocycloalkyl, halo-(3- to 6-membered heterocycloalkyl), OH, oxo, CO2H, CO2—C1-4-alkyl, CONHCH2CO2H, CONH(CH2)2SO3H, SO3H, O—C1-4-alkyl and O-halo-C1-4-alkyl;
R75 is independently selected from C1-4-alkyl, 3- to 6-membered cycloalkyl, 3- to 6-membered heterocycloalkyl, 6-membered aryl and 5- to 6-membered heteroaryl,
wherein alkyl, cycloalkyl, heterocycloalkyl, aryl and heteroaryl is unsubstituted or substituted with 1 to 3 substituents independently selected from halogen, CN, Me, Et, CHF2, CF3, OH, oxo, CO2H, CONHCH2CO2H, CONH(CH2)2SO3H, SO3H, OMe, OEt, OCHF2, and OCF3;
X is independently selected from O, NR51, S(O)n, S(═NR71), S(O)(═NR71) and S(═NR71)2;
Y is independently selected from a bond, O, NR51, S(O)n, S(═NR71), S(O)(═NR71) and S(═NR71)2;
n is independently selected from 0 to 2;
and with the proviso, that the following structures are excluded:
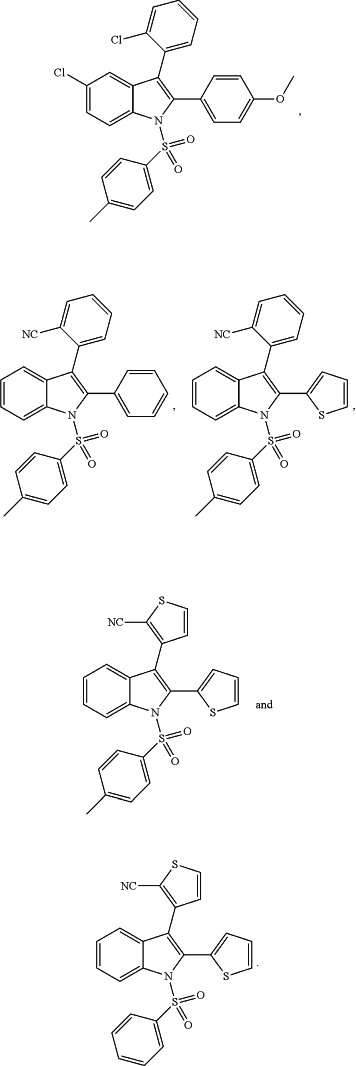 |