| CPC C07D 401/14 (2013.01) [C07D 405/14 (2013.01)] | 18 Claims |
|
1. A compound of the formula I:
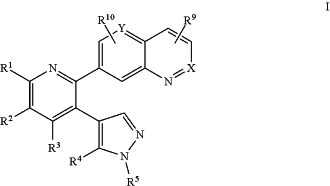 wherein:
X is —N═ or —C(R8)═;
Y is —N═ or —C(R11)═;
R1 is selected from:
(1) hydrogen,
(2) halogen,
(3) —CN,
(4) —C1-6alkyl, which is unsubstituted or substituted with a hydroxy, pyrazolyl, dihydropyranyl, or 1-3 fluoro,
(5) —O—C1-6alkyl, which is unsubstituted or substituted with a hydroxy, or 1-3 fluoro,
(6) cyclopropyl,
(7) —C═CH2,
(8) —C≡CH,
(9) -pyrazolyl, which is unsubstituted or substituted with —C1-6alkyl, and
(10) dihydropyranyl;
R2 is selected from:
(1) hydrogen,
(2) halogen,
(3) —C1-6alkyl,
(4) —CN,
(5) —OC1-6alkyl, and
(6) —SC1-6alkyl;
R3 is selected from:
(1) hydrogen,
(2) chloro,
(3) —C1-6alkyl, and
(4) —OC1-6alkyl;
R4 is selected from:
(1) hydrogen, and
(2) fluoro;
R5 is —C1-6alkyl, which is unsubstituted or substituted with:
(1) fluoro,
(2) hydroxy,
(3) —CN,
(4) keto,
(5) —C1-6alkyl, which is unsubstituted or substituted with hydroxy, methoxy, fluoro, or —C1-6alkyl-fluoro,
(6) —C2-6alkenyl, which is unsubstituted or substituted with fluoro,
(7) —OC1-6alkyl, which is unsubstituted or substituted with —C1-6alkyl, hydroxy, methoxy, fluoro, or —C1-6alkyl-fluoro,
(8) —C3-4cycloalkyl or C6-10cycloalkyl, which is unsubstituted or substituted with —C1-6alkyl, hydroxy, methoxy, fluoro, or —C1-6alkyl-fluoro,
(9) tetrahydrofuranyl, which is unsubstituted or substituted with —C1-6alkyl, hydroxy, methoxy, or 1-3 fluoro,
(10) tetrahydropyranyl, which is unsubstituted or substituted with —C1-6alkyl, hydroxy, methoxy, or 1-3 fluoro, and
(11) phenyl, which is unsubstituted or substituted with —C1-6alkyl, hydroxy, methoxy, or 1-3 fluoro;
each of R8, R9, R10 and R11 is independently selected from:
(1) hydrogen,
(2) halogen,
(3) —C1-6alkyl, which is unsubstituted or substituted with: hydroxy, —OC1-6alkyl, cyclopropyl, cyclobutyl, or 1-3 fluoro; phenyl, which is unsubstituted or substituted with hydroxy, —C1-6alkyl or fluoro; or pyridyl, which is unsubstituted or substituted with hydroxy, —C1-6alkyl or fluoro,
(4) —OC1-6alkyl, which is unsubstituted or substituted with a hydroxy, —OC1-6alkyl, cyclopropyl, cyclobutyl, or 1-3 fluoro,
(5) —C3-6cyclolkyl, which is unsubstituted or substituted with a hydroxy, methoxy, or 1-3 fluoro, and
(6) —CN;
or a pharmaceutically acceptable salt thereof.
|