| CPC A61L 31/16 (2013.01) [A61F 2/82 (2013.01); A61K 31/337 (2013.01); A61K 31/436 (2013.01); A61L 31/10 (2013.01); A61L 33/18 (2013.01); B05D 1/002 (2013.01); B05D 1/02 (2013.01); B05D 3/0486 (2013.01); A61L 2300/256 (2013.01); A61L 2300/258 (2013.01); A61L 2300/412 (2013.01); A61L 2300/416 (2013.01); A61L 2300/604 (2013.01); A61L 2420/08 (2013.01)] | 21 Claims |
|
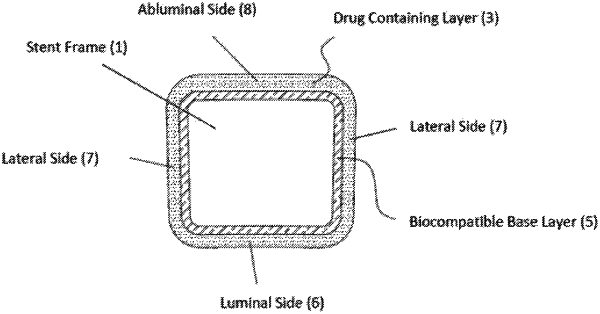
1. A drug eluting stent, comprising:
a stent framework comprising a luminal side, a lateral side, and an abluminal side;
a single biodegradable drug-containing layer comprising one or more polymers coating the luminal side, the lateral side, and the abluminal side of the stent, each side of the polymer coating having a thickness;
a drug embedded in at least one side of the single drug-containing layer; and
a biocompatible base layer disposed over the stent framework and supporting the single drug-containing layer,
wherein:
(i) the degradation of one or more polymers on the luminal side and the lateral side is faster than the degradation of one or more polymers on the abluminal side of the single drug-containing layer;
(ii) the single drug-containing layer releases the drug completely within 30 days after stent implantation within a vessel;
(iii) a ratio between the thickness of the single drug-containing layer on the luminal side and the thickness of the single drug-containing layer on the abluminal side is between 2:3 and 1:7;
(iv) the thickness of the single drug-container layer on the luminal side and the thickness of the drug-container layer on the lateral side are the same as each other; and
(v) wherein the single drug-containing layer on the luminal and lateral sides releases the drug within 10 to 20 days, and releases the drug faster than the single drug-containing layer on the abluminal side.
|