| CPC A61K 9/5123 (2013.01) [A61K 9/5146 (2013.01); A61K 31/7088 (2013.01); A61K 45/06 (2013.01); A61K 48/0033 (2013.01); C12N 15/113 (2013.01); C12N 2320/32 (2013.01)] | 13 Claims |
|
1. A nanoparticle composition comprising a lipid component comprising
an ionizable lipid,
a phospholipid,
a cholesterol, and
a cholesterol derivative; wherein the cholesterol derivative is
(a) β-sitosterol or campesterol; or
(b) a compound of the formula
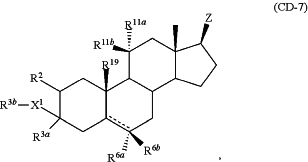 or a salt thereof, wherein Ra is C1-6 alkyl optionally substituted with OH or halo; Rb is C1-6 alkyl optionally substituted with OH or halo; and D1 is H or F,
wherein the molar ratio between the cholesterol and cholesterol derivative is between about 1:100 and 100:1.
|