| CPC A61K 51/1282 (2013.01) [A61N 5/1001 (2013.01); A61K 9/0004 (2013.01); A61K 9/0009 (2013.01); A61K 9/0024 (2013.01)] | 49 Claims |
|
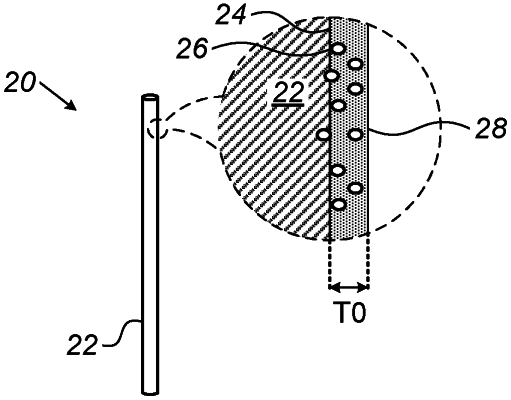
1. A brachytherapy device, comprising:
a seed base adapted for being at least partially introduced into a body of a subject; and
a plurality of radionuclide atoms of a first alpha-emitting isotope, coupled to the seed base, such that at least 0.1%, but not more than 25% of the plurality of radionuclide atoms coupled to the seed base leave the seed base in 24 hours, without radioactive decay,
wherein daughter nuclei of the plurality of radionuclide atoms are released from the seed base upon radioactive decay.
|