| CPC A61K 36/75 (2013.01) [A61K 9/08 (2013.01); A61K 9/1075 (2013.01); A61K 31/192 (2013.01); A61K 31/202 (2013.01); A61K 31/728 (2013.01); A61K 35/74 (2013.01); A61K 36/28 (2013.01); A61K 36/48 (2013.01); A61K 36/54 (2013.01); A61K 47/02 (2013.01); A61K 47/26 (2013.01); A61K 47/34 (2013.01); A61P 27/04 (2018.01)] | 16 Claims |
|
1. A microemulsion composition for treating the eye comprising:
i) a safe and effective amount of a compound and/or extract having retinol-like properties and/or benefits for use in treating the eye selected from one or more of:
botanical extracts from plants of the genus Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts of the genus Actinomyces; and compounds of Formula (I):
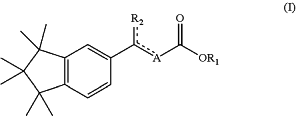 wherein—
the dotted lines represent simple or double bound; optionally one of the dotted line is a double bound;
R1 represent an H, a carbonated chain, linear, cyclic, or branched, saturated or unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated or unsaturated, comprising from 1 to 20 carbon atoms;
optionally a methyl (—CH3) or methylene (═CH2) moiety; a carbonated chain, linear, ringed, or branched, saturated or unsaturated, comprising from 1 to 20 carbon atoms; optionally from 1 to 10 carbon atoms;
optionally 6 carbon atoms; optionally an aromatic moiety, optionally a phenyl moiety; optionally 2-methyl-prop-1,3-diene;
ii) an ophthalmologically acceptable carrier,
wherein the microemulsion droplets or particles have a maximum dimension of less than 1,500 Å.
|