| CPC A61K 31/497 (2013.01) [A61K 31/4545 (2013.01); A61K 31/501 (2013.01); A61K 31/506 (2013.01); A61K 45/06 (2013.01); A61P 31/06 (2018.01); C07D 401/04 (2013.01)] | 4 Claims |
|
1. A combination comprising:
a compound, wherein the compound is 4,4,4-trifluoro-1-(4-(2-(trifluoromethyl)pyridin-4-yl)piperidin-1-yl)butan-1-one having the following structure:
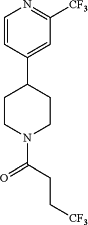 or a pharmaceutically acceptable salt thereof;
at least one anti-tuberculosis agent selected from isoniazid, rifampin, pyrazinamide, ethambutol, moxifloxacin, rifapentine, clofazimine, ethionamide, prothionamide, isoxyl, thiacetazone, rifabutin, a diarylquinoline, nitroimidazo-oxazine PA-824, delamanid (OPC-67683), an oxazolidinone, EMB analogue SQ109, OPC-167832, GSK3036656 (also known as GSK070), GSK2556286, GSK3211830, a benzothiazinone, an azaindole, a dinitrobenzamide, and a beta-lactam; and
at least one anti-viral agent selected from zidovudine, didanosine, lamivudine, zalcitabine, abacavir, stavudine, adefovir, adefovir dipivoxil, fozivudine, todoxil, emtricitabine, alovudine, amdoxovir, elvucitabine, nevirapine, delavirdine, efavirenz, loviride, immunocal, oltipraz, capravirine, lersivirine, GSK2248761, TMC-278, TMC-125, etravirine, saquinavir, ritonavir, indinavir, nelfinavir, amprenavir, fosamprenavir, brecanavir, darunavir, atazanavir, tipranavir, palinavir, lasinavir, enfuvirtide, T-20, T-1249, PRO-542, PRO-140, TNX-355, BMS-806, BMS-663068, BMS-626529, 5-Helix, raltegravir, elvitegravir, GSK1349572, GSK1265744, vicriviroc (Sch-C), Sch-D, TAK779, maraviroc, TAK449, didanosine, tenofovir, lopinavir, and darunavir.
|