| CPC A01N 1/021 (2013.01) [A01N 1/02 (2013.01); A01N 1/0247 (2013.01); A61J 1/00 (2013.01); A61M 1/16 (2013.01); C12M 29/12 (2013.01); C12M 29/14 (2013.01); C12M 41/16 (2013.01); A01N 1/0278 (2013.01); A61M 1/3621 (2013.01); A61M 1/38 (2013.01); A61M 2210/1039 (2013.01)] | 9 Claims |
|
1. A system for maintaining an extracorporeal organ, tissue or bioengineered graft, comprising
an extracorporeal chamber to contain and support the organ, tissue or bioengineered graft; wherein the chamber comprises a time- and temperature-controlled humidifier and misting spray onto the organ, tissue or bioengineered graft and into the chamber to maintain a humid environment within the chamber; and
an extracorporeal cross-circulation circuit to connect the organ, tissue or bioengineered graft with a host organism for cross-circulation of blood or plasma, comprising
fluid conduits providing flow of the blood or plasma between a vascular system of the host organism to vasculature of the organ, tissue or bioengineered graft
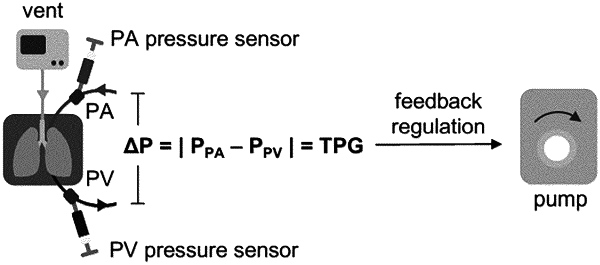
an arterial pressure sensor for sensing extracorporeal in-flow line pressure;
a venous pressure sensor for sensing extracorporeal out-flow line pressure; and
a controller configured to control a circulating pump to regulate blood or plasma flow within a range of 5% to 10% of cardiac output of the host organism based on the trans-organ pressure difference between arterial and venous pressure measured by the arterial pressure sensor and the venous pressure sensor.
|