| CPC H01M 10/54 (2013.01) [B09B 3/00 (2013.01); B09B 5/00 (2013.01); C01G 45/02 (2013.01); C01G 49/02 (2013.01); C22B 1/02 (2013.01); C22B 3/06 (2013.01); C22B 3/44 (2013.01); C22B 7/00 (2013.01); C22B 7/007 (2013.01); C22B 15/00 (2013.01); C22B 15/0086 (2013.01); C22B 21/00 (2013.01); C22B 23/00 (2013.01); C22B 23/0415 (2013.01); C22B 26/12 (2013.01); H01M 10/0525 (2013.01); Y02P 10/20 (2015.11); Y02W 30/84 (2015.05)] | 20 Claims |
|
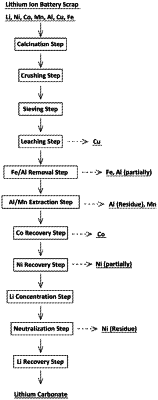
1. A method for treating lithium ion battery scrap containing Li, Ni, Co, Mn, Al, Cu and Fe, the method comprising carrying out a calcination step, a crushing step and a sieving step in this order, and after the steps, the method comprising:
a leaching step of leaching the lithium ion battery scrap by adding it to an acidic solution to leave at least a part of Cu as a solid;
a Fe/Al removal step comprising allowing a leached solution obtained in the leaching step to pass through a Fe removal process for separating and removing Fe under a condition at pH of 3.0 to 4.0 and an oxidation-reduction potential (ORPvsAg/AgCl) of 500 mV or more by addition of an oxidizing agent and an Al removal process for separating and removing a part of Al under a condition at pH of 4.0 to 6.0 and an oxidation-reduction potential (ORPvsAg/AgCl) of −500 mV to 100 mV by neutralization in any order;
an Al/Mn extraction step of extracting and removing a residue of Al and Mn from a separated solution obtained in the Fe/Al removal step by solvent extraction;
a Co recovery step of extracting and back-extracting Co from a first extracted solution obtained in the Al/Mn extraction step by solvent extraction and recovering the Co by electrolytic winning;
a Ni recovery step of extracting and back-extracting, by solvent extraction, a part of Ni from a second extracted solution obtained by the solvent extraction in the Co recovery step and recovering the Ni by electrolytic winning;
a Li concentration step of extracting and back-extracting, by solvent extraction, a residue of Ni and Li from a third extracted solution obtained by the solvent extraction in the Ni recovery step and repeating the operations of the extracting and the back-extracting to concentrate Li; and
a Li recovery step of carbonating Li in a Li concentrated solution obtained in the Li concentration step to recover the Li as lithium carbonate.
|