| CPC H01M 10/0569 (2013.01) [H01M 4/386 (2013.01); H01M 10/0525 (2013.01); H01M 10/0567 (2013.01); H01M 10/0568 (2013.01); H01M 2004/027 (2013.01); H01M 2300/0028 (2013.01)] | 14 Claims |
|
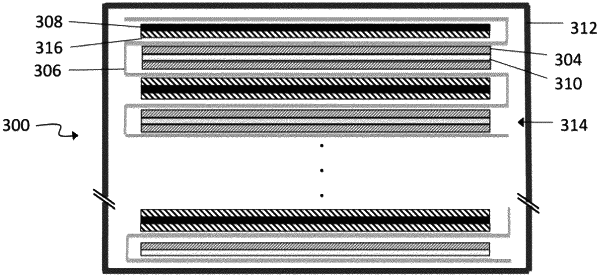
1. An energy storage device comprising:
a first electrode;
a second electrode;
a separator between the first electrode and the second electrode; and
an electrolyte system comprising:
a phosphorus based compound;
a linear carbonate;
a cyclic carbonate: and
a Li-containing salt;
wherein at least one of the first electrode and the second electrode is a Si-base electrode; and
wherein the phosphorus based compound is selected from formulae (B), (C), (D), or combinations thereof:
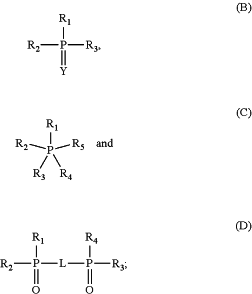 wherein:
each R1, R2, R3, R4, and R5 are independently selected from the group consisting of —R0, —OR, —NR′R′, —NHR′, —SR″, provided that not all of R1, R2, and R3 in Formula (A) or (B) are —R0, provided that not all of R1, R2, R3, R4, and R5 in Formula (C) are —R0, and provided that not all of R1, R2, R3, and R4 in formula (D) are —R0;
each R0 is selected from the group consisting of —CN, —NCO, a C1-C6 alkyl optionally substituted by —CN, and a C2-C6 alkenyl;
each R is selected from the group consisting of hydrogen, a negative charge, a C1-C20 alkyl optionally substituted by —F, —CN, —CF3 or heterocycloalkyl, a C1-C20 heteroalkyl with 1 or more O heteroatom optionally substituted by —F, —CN, —CF3 or heterocycloalkyl, a C2-C20 alkenyl, a C2-C20 heteroalkenyl with 1 or more O heteroatom, a C2-C20 alkynyl, a C2-C20 heteroalkynyl with 1 or more O heteroatom, —Si(CH3)3, a cycloalkyl, a heterocycloalkyl,
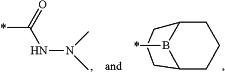 wherein * indicates bonding to O;
each R′ is selected from the group consisting of a C1-C10 alkyl optionally substituted by —F, —CN or heterocycloalkyl, a C1-C10 heteroalkyl with 1 or more O heteroatom optionally substituted by —F, —CN or heterocycloalkyl, a C2-C10 alkenyl, a C2-C10 heteroalkenyl with 1 or more O heteroatom, a C2-C10 alkynyl, and a C2-C10 heteroalkynyl with 1 or more O heteroatom; or R′ is taken together with an adjacent R′ and linked by an N to which one R′ is attached to form a heterocycloalkyl optionally substituted by C1-C3 alkyl;
each R″ is selected from the group consisting of a C1-C20 alkyl optionally substituted by —F, —CN, —CF3 or heterocycloalkyl, a C1-C20 heteroalkyl with 1 or more O heteroatom optionally substituted by —F, —CN, —CF3 or heterocycloalkyl, a C2-C20 alkenyl, a C2-C20 heteroalkenyl with 1 or more O heteroatom, a C2-C20 alkynyl, and a C2-C20 heteroalkynyl with 1 or more O heteroatom;
Y is
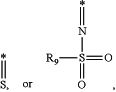 wherein * indicates bonding to P and R9 is alkyl; and
L is a C1-C8 alkyl, or a C1-C8 heteroalkyl with one or more O heteroatom.
|