| CPC G01N 33/533 (2013.01) [G01N 33/505 (2013.01); G06F 17/18 (2013.01); C07K 16/2896 (2013.01); C07K 16/40 (2013.01); G01N 2333/70596 (2013.01)] | 12 Claims |
|
1. A method for the in vitro determination of the potency of an anti-CD26 ligand comprising the following steps:
a) incubating at 37° C. or at room temperature a population of human T lymphocytes expressing the CD26 receptor in a percentage higher than 75% with an anti-CD26 ligand at a concentration ranging from 0.001 μg/ml to 150 μg/ml;
b) contacting said human T lymphocytes with an anti-CD26 antibody conjugated to a fluorochrome; wherein the anti-CD26 antibody recognizes a different CD26 epitope from the epitope recognized by the anti-CD26 ligand used in step a);
c) determining the mean fluorescence intensity (MFI) of CD26 measured for the sample of cells treated with the anti-CD26 ligand (MFIT) and the MFI value of untreated cells (MFINT) by means of cytofluorimetric analysis;
d) evaluating the internalization percentage of the CD26 receptor (% int CD26) or RFI calculated according to the following formula:
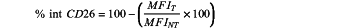 wherein if the value of % intCD26 is:
less than 20% it indicates a low potency of the anti-CD26 ligand;
ranging from 20% to 30% it indicates a medium potency of the anti-CD26 ligand; and
higher than 30% indicates a high potency of the anti-CD26 ligand.
|