| CPC C07F 15/0086 (2013.01) [H10K 85/346 (2023.02)] | 17 Claims |
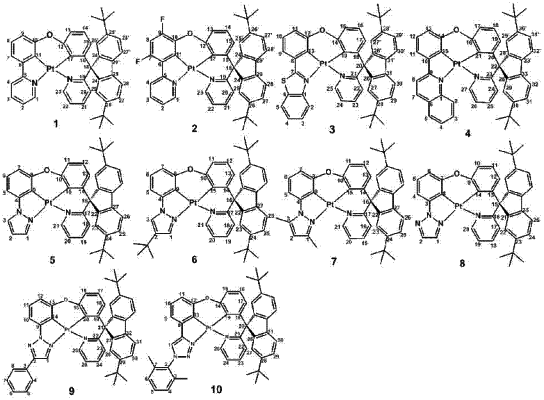
|
1. An asymmetric metal complex, comprising the structure:
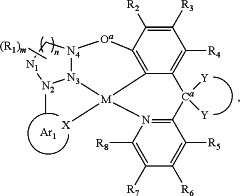 where M is Pt, Ir, W, Fe, Ru, Ni, Cu, Au or Zn; where Ar1 is a substituted or unsubstituted aromatic or heteroaromatic ring where X is N or other electron pair donor;
 is
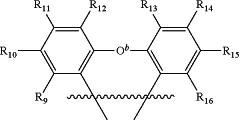 and forms a spiro unit with Ca, where Ca is a bridging atom and can be independently: nitrogen, carbon, boron, phosphorus, silicon, sulfur, or a combination thereof and O b is a bond, O, S, NR17, B R17, PR17, CR17R18, or SiR17R18; where Oa is O, S, NR19, B R19, PR19, CR19R20, or SiR19R20; where N1, N2 and N4 are independently unsubstituted or substituted boron, carbon, nitrogen, oxygen, silicon, germanium, phosphorous, sulphur or selenium and n is 1 to 3; where N3 is carbon, boron, nitrogen, oxygen, silicon, phosphorous, sulphur or selenium; and the ring containing N1, N2, N3 and N4 is a saturated, unsaturated or aromatic ring; where R1 is independently hydrogen, substituted or unsubstituted alkyl, alkenyl, alkynyl, aryl, heteroaryl, cycloalkyl, heterocyclyl, amino, nitro, hydroxyl, halogen, thio, alkoxy, haloalkyl, arylalkane or arylalkene; where R2, R3, R4, R5, R6, R7, and R8 are independently hydrogen, substituted or unsubstituted alkyl, alkenyl, alkynyl, aryl, heteroaryl, cycloalkyl, heterocyclyl, amino, nitro hydroxyl, halogen, thio, alkoxy, haloalkyl, arylalkane or arylalkene; where R9, R10, R11, R12, R13, R14, R15, and R16 are independently hydrogen, halogen, hydroxyl, an unsubstituted alkyl, a substituted alkyl, cycloalkyl, an unsubstituted aryl, a substituted aryl, acyl, alkoxy, acyloxy, amino, nitro, acylamino, aralkyl, cyano, carboxyl, thio, styryl, aminocarbonyl, carbamoyl, aryloxycarbonyl, phenoxycarbonyl, or an alkoxycarbonyl group and where a pair of adjacent R groups can independently form a 5-8 membered aryl or arylalkyl ring which may be interrupted one or more times with O, S, N, or NR21; and where R17, R18, R19, R20, and R21 are independently alkyl, alkenyl, alkynyl, aryl, heteroaryl, cycloalkyl, heterocyclyl, haloalkyl, arylalkane or arylalkene.
|
|
9. A tetradentate ligand, comprising the structure:
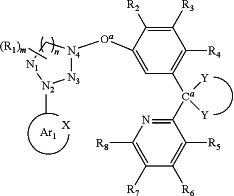 where Ar1 is a substituted or unsubstituted aromatic or heteroaromatic ring where X is N or other electron pair donor;
 is
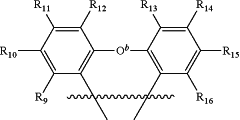 and forms a spiro unit with Ca, where Ca is carbon or silicon and O b is a bond, O, S, NR17, B R17, PR17, CR17R18, or SiR17R18, where Oa is O, S, NR19, B R19, PR19, CR19R20, or SiR19R20, where N1, N2 and N4 are independently unsubstituted or substituted boron, carbon, nitrogen, oxygen, silicon, germanium, phosphorous, sulphur or selenium and n is 1 to 3; where N3 is carbon, boron, nitrogen, oxygen, silicon, germanium, phosphorous, sulphur or selenium; and the ring containing N1, N2, N3 and N4 is a saturated, unsaturated or aromatic ring; where R1 is independently hydrogen, substituted or unsubstituted alkyl, alkenyl, alkynyl, aryl, heteroaryl, cycloalkyl, heterocyclyl, amino, nitro, hydroxyl, halogen, thio, alkoxy, haloalkyl, arylalkane or arylalkene; where R2, R3, R4, R5, R6, R7, and R8 are independently hydrogen, substituted or unsubstituted alkyl, alkenyl, alkynyl, aryl, heteroaryl, cycloalkyl, heterocyclyl, amino, nitro hydroxyl, halogen, thio, alkoxy, haloalkyl, arylalkane or arylalkene; where R9, R10, R11, R12, R13, R14, R15, and R16 are independently hydrogen, halogen, hydroxyl, an unsubstituted alkyl, a substituted alkyl, cycloalkyl, an unsubstituted aryl, a substituted aryl, acyl, alkoxy, acyloxy, amino, nitro, acylamino, aralkyl, cyano, carboxyl, thio, styryl, aminocarbonyl, carbamoyl, aryloxycarbonyl, phenoxycarbonyl, or an alkoxycarbonyl group and where a pair of adjacent R groups can independently form a 5-8 membered aryl or arylalkyl ring which may be interrupted one or more times with O, S, N, or NR21; and where R17, R18, R19, R20, and R21 are independently alkyl, alkenyl, alkynyl, aryl, heteroaryl, cycloalkyl, heterocyclyl, haloalkyl, arylalkane or arylalkene, and wherein when Ar1 is pyridyl or isoquinyl or R1 is halogen then at least one of R10 and R15 is not t-butyl when R9, R11, R12, R13, R14, and R16 are hydrogen.
|