| CPC A61K 31/4709 (2013.01) [A61K 31/42 (2013.01); A61K 31/436 (2013.01); A61K 31/52 (2013.01); A61K 31/5377 (2013.01); A61K 31/573 (2013.01); A61K 35/17 (2013.01); A61K 38/13 (2013.01); A61K 38/1774 (2013.01); A61K 38/1793 (2013.01); A61K 39/3955 (2013.01); A61P 37/06 (2018.01); C07D 401/12 (2013.01)] | 29 Claims |
|
1. A compound according to Formula I:
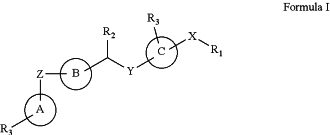 or a pharmaceutically acceptable enantiomer, or salt thereof, wherein:
rings A, B, and C are independently phenyl, pyridine, pyrimidine, pyridazine, pyrazine, triazine, quinoline, quinazoline, isoquinoline, naphthalene, naphthyridine, indole, isoindole, cinnoline, phthalazine, quinoxaline, pteridine, purine, or benzimidazole;
R1 is selected from —(C1-C30)-alkyl, —(C3-C12)-cycloalkyl, —(C3-C12)-heterocycloalkyl, —(C6-C20)-aryl, or —(C3-C20)-heteroaryl groups optionally substituted by one or more substituents selected from —(C1-C12)-alkyl, —(C3-C12)-cycloalkyl, —(C3-C12)-heterocycloalkyl, —O—(C1-C12)-alkyl, —O—(C3-C12)-cycloalkyl, —S—(C1-C12)-alkyl, —S—(C3-C12)-cycloalkyl, —COO—(C1-C12)-alkyl, —COO—(C3-C12)-cycloalkyl, —CONH—(C1-C12)-alkyl, —CONH—(C3-C12)-cycloalkyl, —CO—(C1-C12)-alkyl, —CO—(C3-C12)-cycloalkyl, —N—[(C1-C12)-alkyl]2, —COOH, —OH, —SH, —SO3H, —CN, —NH2, or a halogen;
X, Y, and Z are independently —O, —NH, —S, or —N—(C1-C30)-alkyl;
R2 is ═O, —OH, —SO2, —SO, or —SOCH3;
R3 on ring A is —(C1-C30)-alkyl, —(C3-C12)-cycloalkyl, —(C3-C12)-heterocycloalkyl, —(C6-C20)-aryl, —(C3-C20)-heteroaryl, —O—(C1-C12)-alkyl, —O—(C3-C12)-cycloalkyl, —S—(C1-C12)-alkyl, —S—(C3-C12)-cycloalkyl, —COO—(C1-C12)-alkyl, —COO—(C3-C12)-cycloalkyl, —CONH—(C1-C12)-alkyl, —CONH—(C3-C12)-cycloalkyl, —CO—(C1-C12)-alkyl, —CO—(C3-C12)-cycloalkyl, —N—[(C1-C12)-alkyl]2, (C6-C20)-aryl-(C1-C12)-alkyl, (C3-C20)-heteroaryl-(C1-C12)-alkyl, —COOH, —OH, —SH, —SO3H, —CN, —NH2, or a halogen; and
R3 on ring C is hydrogen, —(C1-C30)-alkyl, —(C3-C12)-cycloalkyl, —(C3-C12)-heterocycloalkyl, —N—[(C1-C12)-alkyl]2, —COOH, —OH, —SH, —SO3H, —CN, —NH2, or a halogen,
with the proviso that:
(i) when R1 is unsubstituted pyridine or N-substituted pyridine by -(C1)-alkyl, X, Y, Z are —NH, R2 is ═O, A is quinoline or pyridine, B is phenyl or pyridine, C is phenyl, R3 on ring C is hydrogen, then R3 on ring A is not —COO—(C1)-alky, not —CO—(C1)-alkyl, not —N—[(C1)-alkyl]2, not —COOH, not —CN, and not —F;
(ii) when the compound has the structure of Formula III,
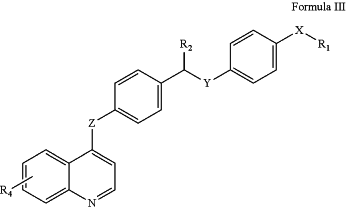 R1 is unsubstituted pyridine, N-substituted pyridine by -(C1-C3)-alkyl, or pyrimidine substituted by -(C1)-alkyl and —NH2, X, Y, Z are —NH, R2 is ═O, then R4 is not halogen, not —(C1)-alkyl, not —O—(C1)-alkyl, not —NH2, and not —N—[(C1)-alkyl]2;
(iii) when R1 is unsubstituted pyridine, unsubstituted quinoline, quinoline substituted by —NH2, —NO2, or —N—[(C1)-alkyl]2, or pyrimidine substituted by -(C1)-alkyl and —NH2, X, Y, Z are —NH, R2 is ═O, A is pyridine, naphthalene, or pyrimidine, B is phenyl, C is phenyl, R3 on ring C is hydrogen, then R3 on ring A is not —(C1-C2)-alkyl and not —NH2;
(iv) when R1 is acridine, X, Y, Z are —NH, R2 is ═O, A is pyridine or pyrimidine substituted by —(C1)-alkyl and —NH2, B is phenyl, C is phenyl, R3 on ring C is hydrogen, then R3 on ring A is not -(C1)-alkyl; and
(v) when A is pyridine, phenyl, pyrimidine, or quinazoline, B is phenyl or pyridine, C is phenyl, pyridine, or pyrimidine, X is —O or —S, Z is —O, —S, or —NH, Y is —NH, R2 is ═O, R3 on ring A is -(C1)-alkyl, —CN, —CF3, pyrimidine, phenyl, —NH2, halogen, or —CONH-(C1)-alkyl, R3 on ring C is hydrogen, halogen, or -(C1)-alkyl, then R1 is not -(C1-C5)-alkyl, not phenyl, not -(C1-C3)-alkyl substituted by halogen, -(C3)-cycloalkyl, —N-[(C1)-alkyl]2, or -(C4)-heterocycloalkyl.
|