| CPC A61K 31/453 (2013.01) [A61P 25/28 (2018.01)] | 13 Claims |
|
1. A method of improving cognitive function in a subject diagnosed with an age-related cognitive disease, the method comprising administering a therapeutically effective amount of a compound of formula (I):
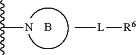 or a pharmaceutically acceptable salt thereof, wherein:
X is N or CH;
n is an integer from 0 to 3;
R1 is selected from halo, —OH, —CN, —(C1-C6)alkyl, —O(C1-C6)alkyl, and —(C3-C6)cycloalkyl;
R2 and R3 are each independently selected from —H and —(C1-C6)alkyl,
wherein R2 and R3 may join to form a 3- to 6-membered ring optionally having from one to three heteroatoms, and
further optionally substituted with one to three groups selected from halo, —OH, (═O), —(C1-C6)alkyl, —O(C1-C6)alkyl, —C(O)O—H, —C(O)(C1-C6)alkyl, and —C(O)NH2;
A is a (4- to 14-membered)N-heterocyclic ring of the following formula:
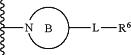 wherein said ring B is: (a) a non-aromatic 4-8 membered monocyclic radical; or (b) a bridged bicyclic radical, a spirocyclic radical, or a 6 to 11-membered fused bicyclic radical,
wherein at least a nonaromatic N-heterocyclic ring of each of said bridged bicyclic radical, spirocyclic radical, or 6 to 11-membered fused bicyclic radical is attached to the carbon atom 1 of the compound of formula (I),
wherein each of said bridged bicyclic radical, spirocyclic radical, and 6 to 11-membered fused bicyclic radical may optionally have an aromatic ring,
wherein said ring B may additionally have from one to three additional ring heteroatoms independently selected from N, O and S; and
wherein said ring B may be further optionally substituted by one to three groups selected from halo, —OH, (═O), —C(O)O—H, —C(O)O—(C1-C6)alkyl, and —(C1-C6)alkyl;
L is absent or a linker selected from —(C1-C6)alkylene-;
each R6 is independently selected from halo, —OR7, —CF3, —CN, —(C1-C6)alkyl, —O(C1-C6)alkyl, —C(O)R7, —C(O)2R7, —C(O)N(R7)2, —N(R7)2, —NHC(O)R7, —NHC(O)N(R7)2, —S(O)2R7, —NH—S(O)2-R7, —(C3-C6)cycloalkyl, -(4- to 14-membered)heterocycloalkyl, —(C6-C10)aryl, and -(5- to 11-membered)heteroaryl,
wherein each of said —(C1-C6)alkyl, —O(C1-C6)alkyl, —(C3-C6)cycloalkyl, -(4- to 14-membered)heterocycloalkyl, —(C6-C10)aryl, and -(5- to 11-membered)heteroaryl of said R6 group is optionally substituted where possible with one to three groups selected from halo, —OH, —CF3, —CN, (═O), —(C1-C6)alkyl, —C(O)O—H, —C(O)O—(C1-C6)alkyl, —NH2, —NH(C1-C6)alkyl, —N((C1-C6)alkyl)2, —S(O)2(C1-C6)alkyl, —(C3-C6)cycloalkyl, -(4- to 14-membered)heterocycloalkyl, —(C6-C10)aryl, and -(5- to 11-membered)heteroaryl; and
each R7 is independently selected from —H, —(C1-C6)alkyl, —(C1-C6)alkyl-OH, —(C1-C6)alkyl-O—(C1-C6)alkyl, —O(C1-C6)alkyl, —(C3-C6)cycloalkyl, —(C3-C6)cycloalkyl-OH, -(4- to 14-membered)heterocycloalkyl, —(C6-C10)aryl, and -(5- to 11-membered)heteroaryl,
wherein each of said R7 groups is optionally substituted where possible with a group selected from —OH, —NH(C1-C6)alkyl, —NHC(O)(C1-C6)alkyl, —C(O)NH2, —S(O)2(C1-C6)alkyl, and -(4- to 14-membered)heterocycloalkyl,
wherein said -(4- to 14-membered)heterocycloalkyl group is optionally substituted where possible with a (═O) group;
and one or more additional active agents.
|