| CPC A61B 5/14532 (2013.01) [A61B 5/14503 (2013.01); A61B 5/4839 (2013.01); A61B 5/6849 (2013.01); A61B 5/7221 (2013.01); A61B 5/742 (2013.01); A61M 5/14244 (2013.01); A61M 5/1723 (2013.01); A61M 2005/14208 (2013.01); A61M 2205/18 (2013.01); A61M 2205/50 (2013.01); A61M 2230/201 (2013.01)] | 20 Claims |
|
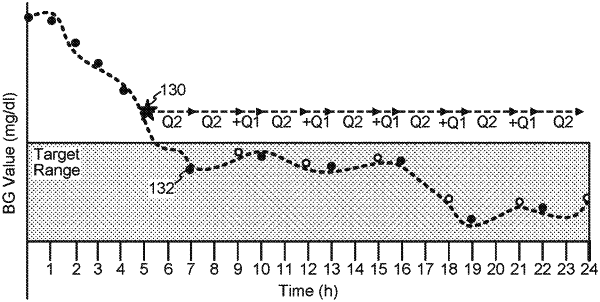
1. A method comprising:
determining a first glucose level reference sample measurement for a patient based on signals from a continuous glucose sensor;
determining to extend a scheduled time for determining a second glucose level reference sample measurement based on a reliability indicator (RI) indicating a reliability of the continuous glucose sensor satisfying a threshold; and
in response to determining to extend the scheduled time, generating signals to provide a message indicating an option to extend the scheduled time for determining the second glucose level reference sample measurement.
|