| CPC H10N 10/855 (2023.02) [C01G 23/006 (2013.01); H10N 10/01 (2023.02); C01P 2002/54 (2013.01); C01P 2002/72 (2013.01); C01P 2004/03 (2013.01); C01P 2006/32 (2013.01); C01P 2006/40 (2013.01)] | 8 Claims |
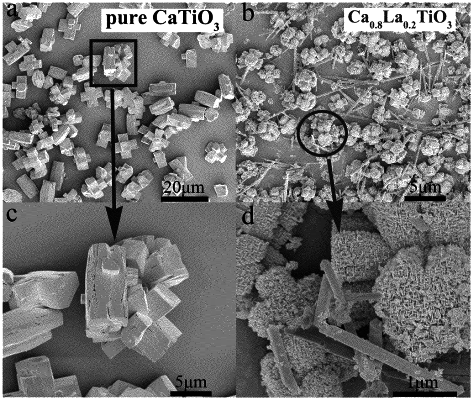
|
1. A method for preparing a CaTiO3-based oxide thermoelectric material, the CaTiO3-based oxide thermoelectric material having a chemical formula of Ca1-xLaxTiO3, where 0<x≤0.4;
the method, comprising:
(1) dissolving La(NO3)3·6H2O in distilled water and stirring for 5-10 minutes, to obtain an aqueous La(NO3)3·6H2O solution;
(2) dissolving CaCl2 in distilled water and stirring for 5-10 minutes, to obtain an aqueous CaCl2 solution;
(3) dissolving NaOH in distilled water and stirring for 5-10 minutes, to obtain an aqueous NaOH solution;
(4) dissolving tetrabutyl titanate in ethylene glycol and stirring for 5-10 minutes, to obtain a solution of tetrabutyl titanate in ethylene glycol;
(5) adding distilled water to the solution of tetrabutyl titanate in ethylene glycol, stirring to obtain a suspension, and adding the aqueous La(NO3)3·6H2O solution, the aqueous CaCl2 solution, and the aqueous NaOH solution in sequence into the suspension, and stirring for 10-15 minutes, to obtain a precursor solution, wherein a molar ratio of the La(NO3)3·6H2O, the CaCl2, the tetrabutyl titanate, and the NaOH is in a range of x: (1−x): 1:10, with the proviso that 0<x≤0.4;
(6) placing the precursor solution into an autoclave, moving the autoclave into a drying box, and keeping at 160-200° C. for 6-24 hours, to obtain a solid product;
(7) mixing glacial acetic acid and distilled water in a glacial acetic acid-to-distilled water volume ratio of 1: (5-15), and stirring for 3-5 minutes, to obtain a mixed solution of glacial acetic acid and distilled water;
(8) adding the solid product into the mixed solution of glacial acetic acid and distilled water, wherein a ratio of the solid product to the mixed solution is in a range of 2-4 g: 100 mL; stirring, and filtering, to obtain a filter cake, washing the filter cake with distilled water for 3 to 5 times, and drying the washed filter cake, to obtain a La-doped CaTiO3 powder; and
(9) sintering the La-doped CaTiO3 powder in a vacuum hot-pressing sintering furnace at 1300 to 1600° C. for 1-3 hours, with a vacuum degree of not more than 0.1 Pa, and a press pressure of 10 to 40 MPa, to obtain the CaTiO3-based oxide thermoelectric material;
wherein steps (1) to (4) are performed in any order; and
there is no time sequence limitation between step (7) and any one of steps (1) to (6).
|