| CPC C07K 7/08 (2013.01) [A61K 38/10 (2013.01); A61K 45/06 (2013.01); A61K 51/088 (2013.01); C07K 7/56 (2013.01); A61K 38/00 (2013.01); Y02A 50/30 (2018.01)] | 6 Claims |
|
1. A method of enhancing, stimulating, and/or increasing an immune response in a subject in need thereof, said method comprising administering to the subject a therapeutically effective amount of a compound of formula (I)
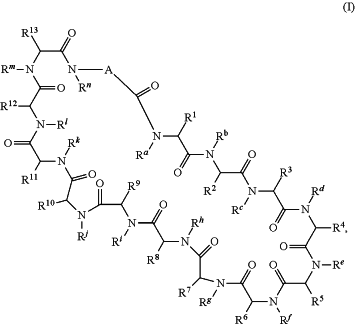 or a pharmaceutically acceptable salt thereof, wherein:
A is
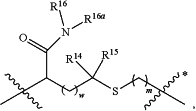 wherein:
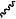 denotes the point of attachment to the carbonyl group and denotes the point of attachment to the carbonyl group and 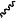 denotes the point of attachment to the nitrogen atom; denotes the point of attachment to the nitrogen atom;m is 1;
w is 0;
R14 and R15 are hydrogen;
R16a is hydrogen;
R16 is —(C(R17a)2)2-X-R30,
X is a chain of between 8 and 46 atoms wherein the atoms are selected from carbon and oxygen and wherein the chain may contain one, two, or three C(O)NH groups embedded therein;
and wherein the chain is optionally substituted with one or two groups independently selected from —CO2H, —C(O)NH2, —CH2C(O)NH2, and —CH2CO2H;
R30 is selected from —CO2H, —C(O)NRwRx, and —CH3 wherein Rw and Rx are hydrogen, provided that when X is all carbon, R30 is other than —CH3;
each R17a is hydrogen,
each of Rc, Rf, Rh, Ri, Rm, and Rn is hydrogen;
Ra, Re, Rj, and Rk, are each independently selected from hydrogen and methyl;
R1 is methyl;
R1 is phenylC1-C3alkyl wherein the phenyl part is optionally substituted with hydroxyl, halo, or methoxy;
R2 is C1-C7alkyl and Rb is methyl; or, R2 and Rb, together with the atoms to which they are attached, form a piperidine ring;
R3 is NRxRy(C1-C7alkyl), NRuRvcarbonylC1-C3alkyl, or carboxyC1-C3alkyl;
R4 and Rd, together with the atoms to which they are attached, form a pyrrolidine ring;
R5 is hydroxyC1-C3alkyl, imidazolylC1-C3alkyl, or NRxRy(C1-C7alkyl);
R6 is carboxyC1-C3alkyl, NRuRvcarbonylC1-C3alkyl, NRxRy(C1-C7alkyl), or C1-C7alkyl;
R7 and Rg, together with the atoms to which they are attached, form a pyrrolidine ring optionally substituted with hydroxy;
R8 and R10 are benzothienyl or indolylC1-C3alkyl optionally substituted with carboxyC1-C3alkyl;
R9 is hydroxyC1-C3alkyl, aminoC1-C3alkyl, or C1-C7alkyl;
R11 is C1-C3alkoxyC1-C3alkyl or C1-C7alkyl;
R12 is C1-C7alkyl or hydroxyC1-C3alkyl; and
R13 is C1-C7 alkyl, carboxyC1-C3alkyl, or —(CH2)3NHC(NH)NH2.
|