| CPC C07D 491/147 (2013.01) [A61P 35/00 (2018.01); C07D 487/14 (2013.01); C07D 495/14 (2013.01)] | 12 Claims |
|
1. A process for synthesizing a compound having the formula I:
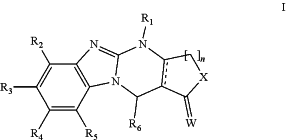 or a pharmaceutically acceptable salt, ester, stereoisomer, or solvate thereof, wherein
 represents a double bond,
n is 1;
W represents oxygen (O);
X represents an oxygen;
R1 represents:
a hydrogen atom;
R2, R3, R4, and R5, which may be the same or different, each represent:
a hydrogen atom;
and
R6 is an aryl or a heteroaryl optionally substituted in one or more positions with one or more of —OCH3, —OH, CH3, —NCH3, —NH2, —NHCOCH3, —F, —Cl, and —Br, the process comprising the following general reaction scheme:
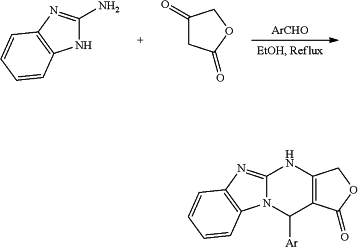 wherein Ar is an aryl or a heteroaryl optionally substituted in one or more positions with one or more of —OCH3, —OH, CH3, —NCH3, —NH2, —NHCOCH3, —F, —Cl, and —Br; and
wherein 2-aminobenzimidazole and tetronic acid are reacted with aldehydes.
|