| CPC C07D 487/04 (2013.01) [A61P 35/00 (2018.01); C07D 471/04 (2013.01); C07D 519/00 (2013.01)] | 20 Claims |
|
1. A method for treating the diseases, disorders, syndromes or conditions associated by inhibition of PRMT5 enzyme to a subject in need thereof, comprising administering to the subject an effective amount of a compound of formula (I), a stereoisomer thereof, or a pharmaceutically acceptable salt thereof, wherein the said diseases, disorders, syndromes or conditions associated by inhibition of PRMT5 enzyme is cancer,
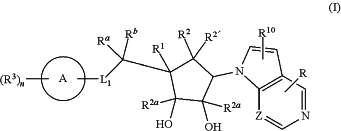 wherein,
L1 is selected from —CRaRb—, —NRa—, S, and O;
Z is selected from CH and N;
Ra and Rb are independently selected at each occurrence from hydrogen, substituted or unsubstituted alkyl, and substituted or unsubstituted cycloalkyl;
ring A is selected from,
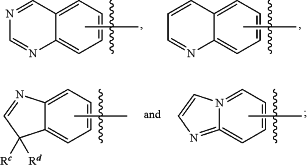 Rc and Rd are selected from substituted or unsubstituted alkyl or together with the carbon atoms to which they are attached form a C3-C6 cycloalkyl ring;
R is selected from —NR4R5, hydrogen, halogen, substituted or unsubstituted alkyl, substituted or unsubstituted alkoxy, substituted or unsubstituted heteroaryl and substituted or unsubstituted cycloalkyl;
R1 and R2 together with the carbon atoms to which they are attached form a bond in order to form a —C═C—; or R1 and R2 together with the carbon atoms to which they are attached form a cyclopropane ring;
R2′ and R2a which may be same or different and are independently selected from hydrogen and substituted or unsubstituted alkyl;
R3 is independently selected at each occurrence from halogen, cyano, nitro, substituted or unsubstituted alkyl, —OR, —NR7R8, substituted or unsubstituted cycloalkyl, —C(O)OH, —C(O)O-alkyl, —C(O)R9, —C(O)NR7R8, —NR7C(O)R9, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and substituted or unsubstituted heterocyclyl;
R4 and R5 are independently selected from hydrogen, substituted or unsubstituted alkyl, and substituted or unsubstituted cycloalkyl;
R6 is selected from hydrogen, substituted or unsubstituted alkyl, and substituted or unsubstituted cycloalkyl;
R7 and R8 are independently selected from hydrogen, substituted or unsubstituted alkyl, and substituted or unsubstituted cycloalkyl;
R9 is selected from substituted or unsubstituted alkyl and substituted or unsubstituted cycloalkyl;
R10 is selected from hydrogen, halogen, and substituted or unsubstituted alkyl;
‘n’ is an integer ranging from 0 to 4, both inclusive;
when an alkyl group is substituted, it is substituted with 1 to 4 substituents independently selected from oxo (═O), halogen, cyano, cycloalkyl, aryl, heteroaryl, heterocyclyl, —OR7a, —C(═O)OH, —C(═O)O(alkyl), —NR8aR8b, —NR8aC(═O)R9a, and —C(═O)NR8aR8b;
when the heteroaryl group is substituted, it is substituted with 1 to 4 substituents independently selected from halogen, nitro, cyano, alkyl, haloalkyl, perhaloalkyl, cycloalkyl, heterocyclyl, aryl, heteroaryl, —OR7a, —NR8aR8b, —NR7aC(═O)R9a, —C(═O)R9a, —C(═O)NR8aR8b, —SO2-alkyl, —C(═O)OH, and —C(═O)O-alkyl;
when the heterocycle group is substituted, it is substituted either on a ring carbon atom or on a ring hetero atom, and when it is substituted on a ring carbon atom, it is substituted with 1 to 4 substituents independently selected from oxo (═O), halogen, cyano, alkyl, cycloalkyl, perhaloalkyl, —OR7a, —C(═O)NR8aR8b, —C(═O)OH, —C(═O)O-alkyl, —N(H)C(═O)(alkyl), —N(H)R8a, and —N(alkyl)2; and when the heterocycle group is substituted on a ring nitrogen, it is substituted with substituents independently selected from alkyl, cycloalkyl, aryl, heteroaryl, —SO2(alkyl), —C(═O)R9a, and —C(═O)O(alkyl); when the heterocycle group is substituted on a ring sulfur, it is substituted with 1 or 2 oxo (═O) group(s);
R7a is selected from hydrogen, alkyl, perhaloalkyl, and cycloalkyl;
R8a and R8b are each independently selected from hydrogen, alkyl, and cycloalkyl; and
R9a is selected from alkyl and cycloalkyl.
|