| CPC C07D 409/12 (2013.01) [A61K 31/415 (2013.01); A61P 25/22 (2018.01); C07D 231/40 (2013.01); C07D 401/12 (2013.01); C07D 403/12 (2013.01); C07D 405/12 (2013.01); C07D 413/12 (2013.01); C07D 417/12 (2013.01)] | 13 Claims |
|
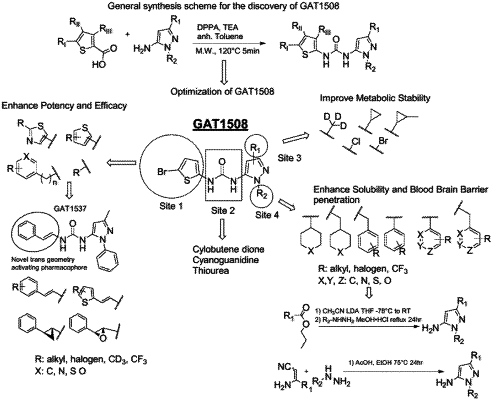
1. A compound for modulation of GIRK channels, the compound having a structure according to Formula I:
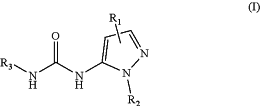 wherein R1 is chosen from hydrogen, halo, methyl, halomethyl, deuteromethyl, cyclopropyl, and cyclopropylmethyl;
wherein R2 is —(CH2)—RC or —RC; RC can be piperidine, thiane, tetrahydropyran, cyclohexyl, or phenyl optionally substituted with one RD, one carbon atom of the phenyl optionally replaced with N, S, or O, wherein RD can be C1-C6 alkyl, halogen, or CF3;
wherein R3 is chosen from —RE—RG, —RF—RG, —RH, and —RI; RE is C2 alkene with trans configuration; RF is cyclopropyl or oxirane; RG is a 5 or 6 membered aromatic ring optionally comprising one or two N, S, or O in place of one or two carbon atoms, the 5 or 6 membered aromatic ring can be optionally substituted with RH; RH can be halogen, CF3, or CD3; RI is a substituted or unsubstituted ring or ring system chosen from thiophene, benzo[b]thiophene, 4,5,6,7-tetrahydrobenzo[b]thiophene, pyrimidine, isoxazole, thiazole, adamantane, benzo[d][1,3]dioxole, and isoquinoline; and wherein RI is optionally substituted with one or more functional groups chosen from halo, and C1-C6 branched or unbranched alkyl optionally substituted with one or more halogens;
or a pharmaceutically-acceptable salt thereof.
|