| CPC C07D 401/14 (2013.01) [A61K 31/404 (2013.01); A61K 31/4178 (2013.01); A61K 31/428 (2013.01); A61K 31/437 (2013.01); A61K 31/454 (2013.01); A61K 31/675 (2013.01); A61K 45/06 (2013.01); A61P 31/14 (2018.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 409/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07F 9/65583 (2013.01)] | 18 Claims |
|
1. A compound of Formula (I), or a pharmaceutically acceptable salt thereof, having the structure:
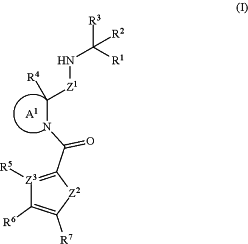 wherein:
Z1 is —C(═O)— or —CH(CF3)—;
Z2 is O, S or NR8, wherein R8 is H or an unsubstituted C1-4 alkyl;
Z3 is N or C, and when Z3 is N, then R5 is absent;
Ring A1 is selected from the group consisting of an unsubstituted or a substituted azetidine, an unsubstituted or a substituted pyrrolidine and an unsubstituted or a substituted piperidine, wherein the azetidine, the pyrrolidine and the piperidine can be optionally substituted with one or more Rx groups independently selected from the group consisting of deuterium, halogen, an unsubstituted or a substituted C1-4 alkyl, an unsubstituted or a substituted C2-4 alkenyl, an unsubstituted or a substituted C1-8 alkoxy, an unsubstituted or a substituted C3-6 cycloalkyl, an unsubstituted or a substituted aryl, an unsubstituted or a substituted heteroaryl, an unsubstituted or a substituted heterocyclyl and an unsubstituted C1-4 haloalkyl, and wherein the azetidine, the pyrrolidine and the piperidine can be connected to a cyclic moiety selected from the group consisting of a monocyclic C3-7 cycloalkyl, a bicyclic C5-9 cycloalkyl, a monocyclic C3-7 cycloalkenyl, a bicyclic C5-9 cycloalkenyl and phenyl, wherein the cyclic moiety is connected to the azetidine, the pyrrolidine and the piperidine in a fused-fashion or a spiro-fashion that can be optionally substituted with one or more moieties independently selected from the group consisting of halogen, an unsubstituted C1-4 alkyl, an unsubstituted C2-4 alkenyl and an unsubstituted or a substituted C3-6 monocyclic cycloalkyl;
R1 is selected from the group consisting of cyano, an unsubstituted or a substituted C2-5 alkynyl, an unsubstituted or a substituted ketoamide, an unsubstituted or a substituted —C(═O)—N-sulfonamido, CH(OH)((P═O)(OR9)2), —C(═O)CH2'O—(P═O)(OR10)2), —C(═O)CH2—O—C(R11A)2—O—((P═O)(OR11B)2), —C(═O)CH2—O—C(R12A)2—O—C(═O)—OR12B and —C(═O)CH2—O—C(R13A)2—O—C(═O)—R13B, wherein each R9, each R10, each R11B and R12B and R13B are independently hydrogen, an unsubstituted C1-6 alkyl, an unsubstituted C2-6 alkenyl, an unsubstituted C1-6 haloalkyl, an unsubstituted or a substituted aryl or an unsubstituted or a substituted aryl(C1-4 alkyl);
each R11A, each R12A and each R13A are independently hydrogen or an unsubstituted C1-4 alkyl;
R2 is hydrogen, deuterium or halogen;
R3 is an unsubstituted or a substituted C-amido(C1-4 alkyl), an unsubstituted or a substituted monocyclic nitrogen-containing heteroaryl(C1-4 alkyl), an unsubstituted or a substituted monocyclic nitrogen-containing heterocyclyl(C1-4 alkyl), an unsubstituted or a substituted bicyclic nitrogen-containing heteroaryl(C1-4 alkyl) or an unsubstituted or a substituted bicyclic nitrogen-containing heterocyclyl(C1-4 alkyl);
R4 is hydrogen, deuterium or halogen;
R5 is selected from the group consisting of hydrogen, deuterium, halogen, an unsubstituted C1-6 alkyl and an unsubstituted C14 haloalkyl; and
R6 and R7 are independently selected from the group consisting of hydrogen, deuterium, halogen, an unsubstituted or a substituted C1-6 alkyl, an unsubstituted or a substituted phenyl, an unsubstituted or a substituted acyl, an unsubstituted or a substituted C-carboxy and an unsubstituted or a substituted sulfonyl; or
R6 and R7 are taken together with the carbon to which R6 and R7 are each attached to form an optionally substituted 4-9 membered saturated or unsaturated ring or ring system that can optionally contain 1 or 2 ring heteroatoms selected from the group consisting of O, N and S.
|