| CPC C01G 53/10 (2013.01) [C01G 53/003 (2013.01); C01P 2006/80 (2013.01)] | 7 Claims |
|
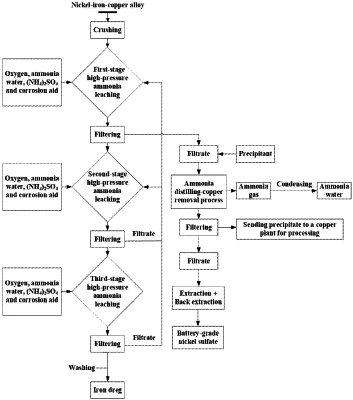
1. A method for preparing nickel sulfate from a nickel-iron-copper alloy, comprising the following steps:
S1: mixing a crushed nickel-iron-copper alloy, ammonia water, ammonium sulfate and a corrosion aid in a high-pressure oxygen environment and performing leaching; the corrosion aid is at least one of ammonium sulfide, persulfate, or ammonium thiosulfate; a pressure of the leaching is 2.5 MPa to 4.0 MPa, and a temperature of the leaching is 50° C. to 65° C.;
S2: performing a solid-liquid separation on a slurry obtained by the leaching in step S1 to obtain a first filtrate and a first residue, adding a precipitant to the first filtrate, then distilling ammonia, and then performing filtration to obtain a nickel-containing leaching solution, wherein the precipitant is at least one of thiosulfate, sodium sulfide, or ammonium sulfide, and wherein each of the first filtrate and the first residue comprises copper; and
S3: adding an extractant into the nickel-containing leaching solution to extract nickel, allowing the system to stand and then separating to obtain a nickel-containing extracted organic phase, and then adding sulfuric acid into the nickel-containing extracted organic phase to perform a back extraction of nickel to obtain a nickel sulfate solution.
|